Radical Acylfluoroalkylation of 1,3-Enynes via N-Heterocyclic Carbene/Photoredox Cooperative Catalysis
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
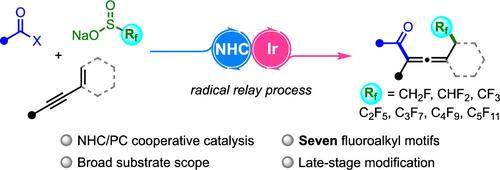
We report a novel three-component radical acylfluoroalkylation of 1,3-enynes by synergistic N-heterocyclic carbene (NHC)/photoredox catalysis toward various fluorinated allenic aryl ketones. This protocol features a broad substrate scope and excellent functional group tolerability, with examples of late-stage modification of drug molecules and natural products. Notably, seven different fluoroalkyl motifs can be introduced to 1,3-enynes, further demonstrating the robustness and generality of this method. The generation of the fluoroalkyl radical from each sulfinate reagent was individually supported by EPR experiments.
通过 N-杂环碳烯/光氧合作催化实现 1,3-炔的自由基酰氟烷基化。
我们报告了一种新颖的三组分 1,3-炔基酰氟烷基化反应,该反应通过 N-杂环碳烯(NHC)/光氧催化协同作用,对各种含氟异烯芳基酮进行酰氟烷基化。该方案具有广泛的底物范围和出色的官能团耐受性,可用于药物分子和天然产品的后期改性。值得注意的是,1,3-烯炔可以引入七种不同的氟烷基基团,这进一步证明了该方法的稳健性和通用性。每种亚磺酸盐试剂产生的氟烷基自由基都得到了 EPR 实验的支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


