Neutrophil-derived PAD4 induces citrullination of CKMT1 exacerbates mucosal inflammation in inflammatory bowel disease
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
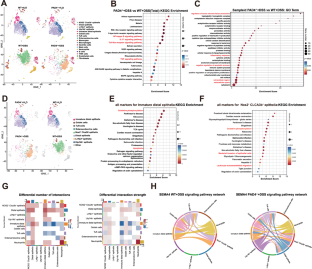
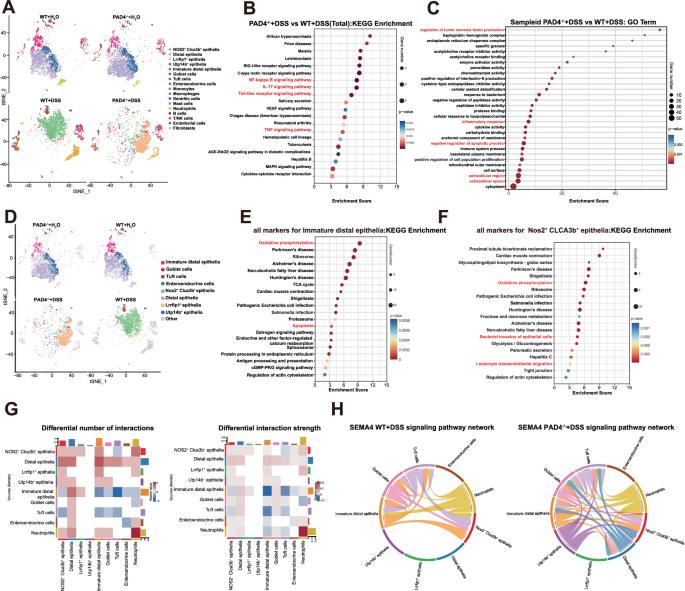
Peptidyl arginine deiminase 4 (PAD4) plays a pivotal role in infection and inflammatory diseases by facilitating the formation of neutrophil extracellular traps (NETs). However, the substrates of PAD4 and its exact role in inflammatory bowel disease (IBD) remain unclear. In this study, we employed single-cell RNA sequencing (scRNA-seq) and substrate citrullination mapping to decipher the role of PAD4 in intestinal inflammation associated with IBD. Our results demonstrated that PAD4 deficiency alleviated colonic inflammation and restored intestinal barrier function in a dextran sulfate sodium (DSS)-induced colitis mouse model. scRNA-seq analysis revealed significant alterations in intestinal cell populations, with reduced neutrophil numbers and changes in epithelial subsets upon PAD4 deletion. Gene expression analysis highlighted pathways related to inflammation and epithelial cell function. Furthermore, we found that neutrophil-derived extracellular vesicles (EVs) carrying PAD4 were secreted into intestinal epithelial cells (IECs). Within IECs, PAD4 citrullinates mitochondrial creatine kinase 1 (CKMT1) at the R242 site, leading to reduced CKMT1 protein stability via the autophagy pathway. This action compromises mitochondrial homeostasis, impairs intestinal barrier integrity, and induces IECs apoptosis. IEC-specific depletion of CKMT1 exacerbated intestinal inflammation and apoptosis in mice with colitis. Clinical analysis of IBD patients revealed elevated levels of PAD4, increased CKMT1 citrullination, and decreased CKMT1 expression. In summary, our findings highlight the crucial role of PAD4 in IBD, where it modulates IECs plasticity via CKMT1 citrullination, suggesting that PAD4 may be a potential therapeutic target for IBD.
中性粒细胞衍生的 PAD4 可诱导 CKMT1 的瓜氨酸化,从而加剧炎症性肠病的粘膜炎症
肽基精氨酸脱氨酶 4(PAD4)通过促进中性粒细胞胞外陷阱(NET)的形成,在感染和炎症性疾病中发挥着关键作用。然而,PAD4 的底物及其在炎症性肠病(IBD)中的确切作用仍不清楚。在这项研究中,我们采用了单细胞 RNA 测序(scRNA-seq)和底物瓜氨酸化图谱来解读 PAD4 在与 IBD 相关的肠道炎症中的作用。我们的研究结果表明,在右旋糖酐硫酸钠(DSS)诱导的结肠炎小鼠模型中,PAD4的缺失减轻了结肠炎症并恢复了肠道屏障功能。scRNA-seq分析揭示了肠道细胞群的显著变化,PAD4缺失后中性粒细胞数量减少,上皮亚群发生变化。基因表达分析强调了与炎症和上皮细胞功能相关的通路。此外,我们还发现携带 PAD4 的中性粒细胞衍生细胞外囊泡 (EV) 被分泌到肠道上皮细胞 (IEC) 中。在 IECs 内,PAD4 在 R242 位点瓜氨酸化线粒体肌酸激酶 1(CKMT1),通过自噬途径导致 CKMT1 蛋白稳定性降低。这种作用会破坏线粒体的平衡,损害肠道屏障的完整性,并诱导 IEC 细胞凋亡。在患有结肠炎的小鼠体内,IEC 特异性 CKMT1 的消耗会加剧肠道炎症和细胞凋亡。对 IBD 患者的临床分析表明,PAD4 水平升高,CKMT1 瓜氨酸化增加,CKMT1 表达减少。总之,我们的研究结果突显了 PAD4 在 IBD 中的关键作用,它通过 CKMT1 瓜氨酸化调节 IECs 的可塑性,这表明 PAD4 可能是 IBD 的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


