TRIM58 downregulation maintains stemness via MYH9-GRK3-YAP axis activation in triple-negative breast cancer stem cells
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
TRIM58 is a member of the TRIM protein family, which possess with E3 ubiquitin ligase activities. Studies have revealed that low expression of TRIM58 plays key roles, has been implicated in the tumor progression of tumor formation due to its reduced expression. However, its role in regulating the stemness of breast cancer stem cells (CSCs) remains unexplored. Here, we found that TRIM58 was underexpressed in TNBC tissues and cells compared to adjacent mucosa tissue, and its downregulation was significantly associated with shorter survival. Overexpression of TRIM58 reduced the proportion of CD44 + /CD24- cells, upregulated differentiation genes, and inhibited stemness-related gene expression in TNBC CSCs. In vitro and in vivo experiments revealed that TRIM58 overexpression in CSCs suppressed tumor sphere formation and tumorigenic capacity. Co-IP results indicated direct interaction between TRIM58 and MYH9, with TRIM58 inducing MYH9 degradation via ubiquitination in differentiated cells. Label-free quantitative proteomics identified GRK3 and Hippo-YAP as downstream targets and signaling pathways of MYH9. TIMER database analysis, immunohistochemistry, western blotting, DNA-protein pulldown experiments, and dual luciferase reporter assays demonstrated that MYH9 regulated GRK3 transcriptional activation in CSCs. In conclusion, elevated TRIM58 expression in CSCs downregulates MYH9 protein levels by promoting ubiquitin-mediated degradation, thereby inhibiting downstream GRK3 transcription, inactivating the YAP stemness pathway, and ultimately promoting CSC differentiation.
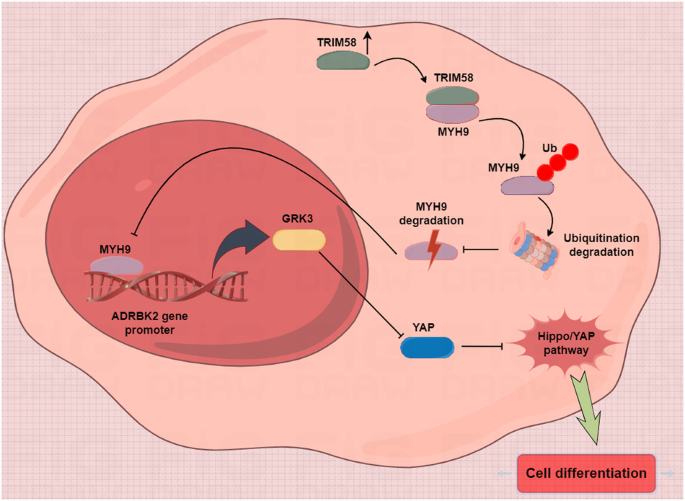
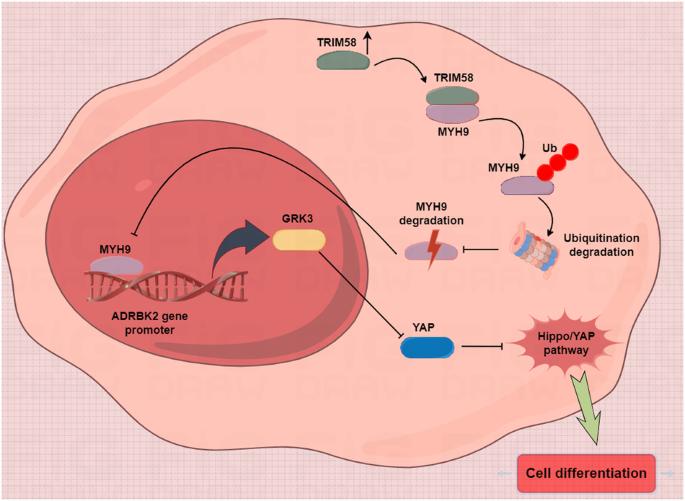
TRIM58 下调可通过激活三阴性乳腺癌干细胞中的 MYH9-GRK3-YAP 轴维持干性。
TRIM58 是 TRIM 蛋白家族的成员,具有 E3 泛素连接酶活性。研究发现,TRIM58的低表达在肿瘤的形成过程中起着关键作用,并因其表达量减少而被认为与肿瘤的进展有关。然而,它在调控乳腺癌干细胞(CSCs)的干性方面的作用仍有待探索。在这里,我们发现与邻近的粘膜组织相比,TRIM58在TNBC组织和细胞中表达不足,其下调与生存期缩短显著相关。TRIM58的过表达降低了CD44 + /CD24-细胞的比例,上调了分化基因,抑制了TNBC CSCs中干性相关基因的表达。体外和体内实验显示,TRIM58 在 CSCs 中的过表达抑制了肿瘤球的形成和致瘤能力。Co-IP结果表明,TRIM58与MYH9之间存在直接相互作用,TRIM58在分化细胞中通过泛素化诱导MYH9降解。无标记定量蛋白质组学发现,GRK3和Hippo-YAP是MYH9的下游靶标和信号通路。TIMER数据库分析、免疫组化、Western印迹、DNA-蛋白牵引实验和双荧光素酶报告实验证明,MYH9可调控CSCs中GRK3的转录激活。总之,TRIM58在CSCs中的表达升高会通过促进泛素介导的降解来下调MYH9蛋白水平,从而抑制下游GRK3的转录,使YAP干性通路失活,最终促进CSC的分化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


