PIONEER REAL Japan: Baseline characteristics of a multicenter, prospective, real-world study of oral semaglutide in adults with type 2 diabetes in clinical practice in Japan
Abstract
Aims/Introduction
PIONEER REAL Japan was a non-interventional, multicenter, prospective study investigating oral semaglutide in adults with type 2 diabetes in routine clinical practice. We report baseline characteristics of participants enrolled in this study.
Materials and Methods
Adults aged ≥20 years with type 2 diabetes but no previous treatment with injectable glucose-lowering medication were enrolled. Participants initiated oral semaglutide at their treating physician's discretion and were followed for 34–44 weeks. Participants were stratified into <75-year-old and ≥75-year-old subgroups.
Results
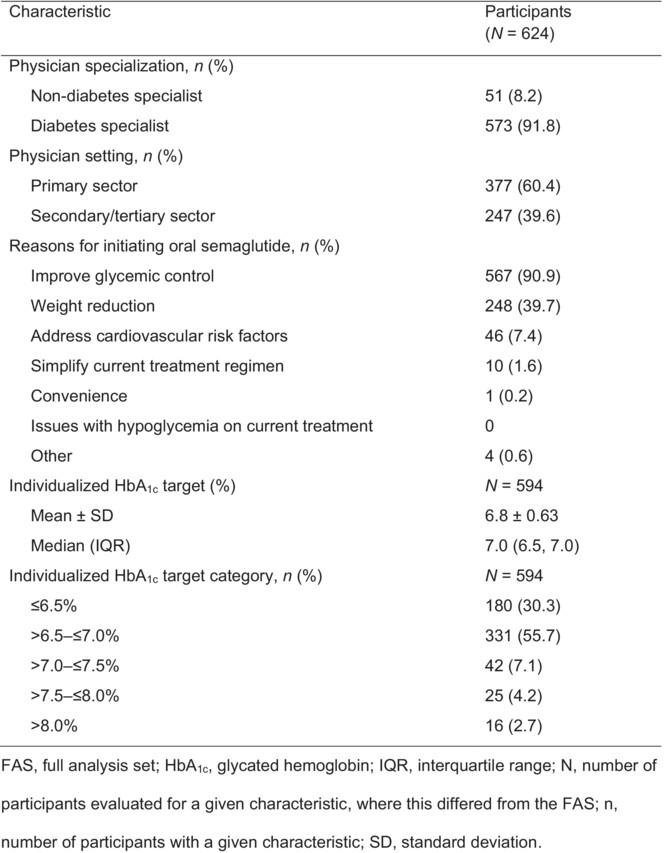
A total of 624 participants initiated the study. The mean (standard deviation) age was 64.1 years (14.1), the mean (standard deviation) body weight was 72.4 kg (16.1), and the mean (standard deviation) body mass index was 27.5 kg/m2 (5.0). Participants had a median (interquartile range) type 2 diabetes duration of 9.3 years (4.2, 15.2) and mean (standard deviation) glycated hemoglobin 7.7% (1.1). Most (75.6%) participants were taking glucose-lowering medications at baseline; the most common was metformin (51.9%). The main reasons for initiating oral semaglutide were glycemic control and weight loss. Most (86.0%) participants had an individualized target for glycemic control of glycated hemoglobin ≤7%. The <75-year-old subgroup was heavier (mean [standard deviation] body mass index 28.6 kg/m2 [5.2] vs 25.1 kg/m2 [3.4]) but had comparable glycated hemoglobin levels (mean [standard deviation] 7.7% [1.2] vs 7.8% [1.0]) to the ≥75-year-old subgroup.
Conclusions
PIONEER REAL Japan describes the characteristics of individuals with type 2 diabetes prescribed oral semaglutide. The baseline characteristics provide insights into Japanese individuals with type 2 diabetes prescribed oral semaglutide in clinical practice.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


