Optimisation of a primary human CAR-NK cell manufacturing pipeline
Abstract
Objectives
Autologous chimeric antigen receptor (CAR) T-cell therapy of B-cell malignancies achieves long-term disease remission in a high fraction of patients and has triggered intense research into translating this successful approach into additional cancer types. However, the complex logistics involved in autologous CAR-T manufacturing, the compromised fitness of patient-derived T cells, the high rates of serious toxicities and the overall cost involved with product manufacturing and hospitalisation have driven innovation to overcome such hurdles. One alternative approach is the use of allogeneic natural killer (NK) cells as a source for CAR-NK cell therapy. However, this source has traditionally faced numerous manufacturing challenges.
Methods
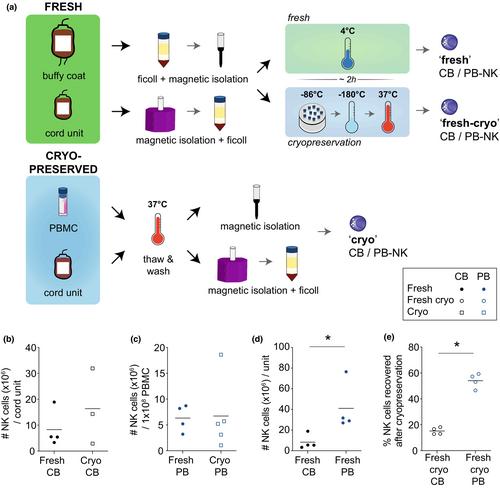
To address this, we have developed an optimised expansion and transduction protocol for primary human NK cells primed for manufacturing scaling and clinical evaluation. We have performed an in-depth comparison of primary human NK cell sources as a starting material by characterising their phenotype, functionality, expansion potential and transduction efficiency at crucial timepoints of our CAR-NK manufacturing pipeline.
Results
We identified adult peripheral blood-derived NK cells to be the superior source for generating a CAR-NK cell product because of a higher maximum yield of CAR-expressing NK cells combined with potent natural, as well as CAR-mediated anti-tumor effector functions.
Conclusions
Our optimised manufacturing pipeline dramatically improves lentiviral transduction efficiency of primary human NK cells. We conclude that the exponential expansion pre- and post-transduction and high on-target cytotoxicity make peripheral blood-derived NK cells a feasible and attractive CAR-NK cell product for clinical utility.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


