Undec-10-enal in the Synthesis of Undec-10-enyl Undec-10-enoate and O- and N-containing Macroheterocycles
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An effective synthesis of four potentially biologically active O- and N-containing 23-, 28-, 30-, and 31-membered macroheterocycles from undec-10-enal was carried out. First, undec-10-enal was converted to undec-10-enyl undec-10-enoate via the Tishchenko disproportionation reaction catalyzed by Al(i-PrO)3. The subsequent Wacker–Tsuji oxidation of undec-10-enyl undec-10-enoate gave 10-oxoundecanyl 10-oxoundecanoate. Finally, the [1+1]-condensation of 10-oxoundecanyl-10-oxoundecenoate with hydrazine hydrate or malonic, glutaric, or adipic acid dihydrazides resulted in the synthesis of four macroheterocycles.
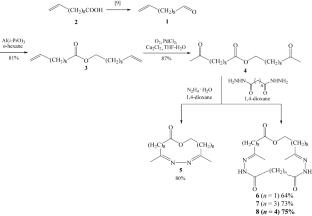
十一-10-烯醛在合成十一-10-烯基十一-10-烯酸酯以及含 O 和 N 的大杂环中的应用
摘要 以十一-10-烯醛为原料,有效合成了四种具有潜在生物活性的含 O 和 N 的 23、28、30 和 31 元大杂环。首先,通过 Al(i-PrO)3 催化的 Tishchenko 歧化反应将十一-10-烯醛转化为十一-10-烯基十一-10-烯酸酯。最后,10-氧代十一烷基-10-氧代癸烯酸与水合肼或丙二酸、戊二酸或己二酸二酰肼进行 [1+1] 缩合,合成了四种大杂环。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


