Tailored SrFeO3-δ for chemical looping dry reforming of methane
IF 7.6
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
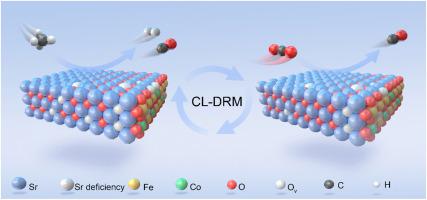
Chemical looping dry reforming of methane (CL-DRM) is a highly efficient process that converts two major greenhouse gases (CH4 and CO2) into syngas ready for the feedstock of liquid fuel production. One of the major obstacles facing this technology now is creating oxygen carriers that are stable and reactive. We fabricated high-performance Sr0.98Fe0.7Co0.3O3-δ perovskite-structured oxygen carrier by combining A-site defects and B-site doping of SrFeO3-δ. During isothermal CL-DRM tests at 850 °C, Sr0.98Fe0.7Co0.3O3-δ achieved 87% CH4 conversion and 94% CO selectivity in the CH4 partial oxidation reaction, followed by a syngas yield of 8.5 mmol/g, and CO yield of 4.2 mmol/g in CO2 decomposition. A-site defect engineering of the perovskite creates abundant oxygen vacancies and enhances oxygen storage capacity (OSC). Co-doping of the B-site of Sr0.98FeO3-δ increases oxygen mobility and CH4/CO2 activation, resulting in high activity in the CL-DRM process. This methodology resulted in high ionic mobility and facilitated the rapid diffusion of oxygen in the bulk phase, thereby increasing the redox properties of SrFeO3-δ. The oxygen carrier exhibits excellent structural stability and regeneration ability in successive redox cycles. This strategy offers a simple but very effective pathway to tailor OSC, oxygen mobility, and oxygen vacancies of perovskite-structured materials for chemical looping or redox-involved processes.
用于甲烷化学循环干式转化的定制 SrFeO3-δ
甲烷化学循环干重整(CL-DRM)是一种高效工艺,可将两种主要温室气体(CH4 和 CO2)转化为合成气,作为生产液体燃料的原料。目前,该技术面临的主要障碍之一是制造稳定且具有活性的氧气载体。我们通过结合 SrFeO3-δ 的 A 位缺陷和 B 位掺杂,制造出了高性能的 Sr0.98Fe0.7Co0.3O3-δ 包晶结构氧载体。在 850 °C 的等温 CL-DRM 试验中,Sr0.98Fe0.7Co0.3O3-δ 在 CH4 部分氧化反应中实现了 87% 的 CH4 转化率和 94% 的 CO 选择性,随后合成气产量为 8.5 mmol/g,CO2 分解的 CO 产量为 4.2 mmol/g。过氧化物的 A 位缺陷工程产生了大量的氧空位,提高了储氧能力(OSC)。Sr0.98FeO3-δ 的 B 位共掺杂增加了氧的迁移率和 CH4/CO2 的活化,从而在 CL-DRM 过程中产生了高活性。这种方法产生了高离子迁移率,促进了氧气在体相中的快速扩散,从而提高了 SrFeO3-δ 的氧化还原特性。氧载体在连续的氧化还原循环中表现出优异的结构稳定性和再生能力。这种策略为定制化学循环或氧化还原过程中的包晶结构材料的 OSC、氧迁移率和氧空位提供了一种简单但非常有效的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemical Engineering
Process Chemistry and Technology, Catalysis, Filtration and Separation
CiteScore
11.60
自引率
0.00%
发文量
58
审稿时长
51 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


