GBP2 inhibits pathological angiogenesis in the retina via the AKT/mTOR/VEGFA axis
Abstract
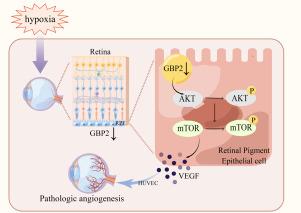
Pathological retinal angiogenesis is not only the hallmark of retinopathies, but also a major cause of blindness. Guanylate binding protein 2 (GBP2) has been reported to be associated with retinal diseases such as diabetic retinopathy and hypoxic retinopathy. However, GBP2-mediated pathological retinal angiogenesis remains largely unknown. The present study aimed to investigate the role of GBP2 in pathological retinal angiogenesis and its underlying molecular mechanism. In this study, we established oxygen-induced retinopathy (OIR) mice model for in vivo study and hypoxia-induced angiogenesis in ARPE-19 cells for in vitro study. We demonstrated that GBP2 expression was markedly downregulated in the retina of mice with OIR and ARPE-19 cells treated with hypoxia, which was associated with pathological retinal angiogenesis. The regulatory mechanism of GBP2 in ARPE-19 cells was studied by GBP2 silencing and overexpression. The regulatory mechanism of GBP2 in the retina was investigated by overexpressing GBP2 in the retina of OIR mice. Mechanistically, GBP2 downregulated the expression and secretion of vascular endothelial growth factor (VEGFA) in ARPE-19 cells and retina of OIR mice. Interestingly, overexpression of GBP2 significantly inhibited neovascularization in OIR mice, conditioned medium of GBP2 overexpressing ARPE-19 cells inhibited angiogenesis in human umbilical vein endothelial cells (HUVECs). Furthermore, we confirmed that GBP2 downregulated VEGFA expression and angiogenesis by inhibiting the AKT/mTOR signaling pathway. Taken together, we concluded that GBP2 inhibited pathological retinal angiogenesis via the AKT/mTOR/VEGFA axis, thereby suggesting that GBP2 may be a therapeutic target for pathological retinal angiogenesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


