Iron catalyzed oxidative Csp3-Csp3 radical coupling reaction between thiohydantoins and O-acetyl oximes for the synthesis of 1,3-dibenzyl-3,4-dihydropyrrolo[2,3-d]imidazole-2(1H)-thione derivatives
引用次数: 0
Abstract
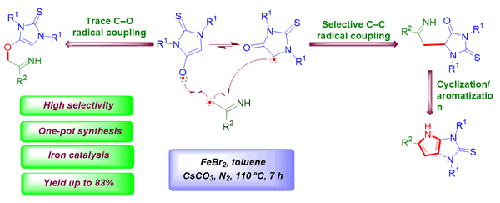
A unified heteroannelation method to achieve the synthesis of 1,3-dibenzyl-3,4-dihydropyrrolo[2,3-d]imidazole-2(1H)-thione derivatives with an iron catalyst is proposed. The annelation reaction is followed by Csp3-Csp3 radical coupling between thiohydantoins and O-acetyl oximes. The synthetic approach enables access to several alkynylated thiohydantoins and O-acetyl oximes with controlled selectivity for Csp3-Csp3 coupling rather than Csp3-O coupling in moderate to higher yield. The optimization study has been carried out by changing catalyst loading, oxidants, solvents, and varying temperatures. Synthesized compounds were characterized through 1H NMR, 13C NMR, and mass spectral studies.
铁催化硫代海因和 O-乙酰肟之间的 Csp3-Csp3 氧化自由基偶联反应,用于合成 1,3- 二苄基-3,4-二氢吡咯并[2,3-d]咪唑-2(1H)-硫酮衍生物
提出了一种在铁催化剂作用下合成 1,3-二苄基-3,4-二氢吡咯并[2,3-d]咪唑-2(1H)-硫酮衍生物的统一异通道法。环化反应之后是硫代海因和 O-乙酰肟之间的 Csp3-Csp3 自由基偶联。通过这种合成方法,可以获得几种炔化硫代海因和 O-乙酰肟,Csp3-Csp3 偶联的选择性可控,而不是 Csp3-O 偶联,产率中等至较高。通过改变催化剂负载量、氧化剂、溶剂和不同温度进行了优化研究。合成的化合物通过 1H NMR、13C NMR 和质谱研究进行了表征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


