Antibiotics administered as continuous intravenous infusion over 24 hours by elastomeric devices to patients treated at home: a study of infusion efficiency
Abstract
Background
Elastomeric infusion devices or ‘Infusors’ are commonly used to administer 24-h continuous intravenous infusions to hospital patients at home, a service which can increase hospital capacity.
Aim
This study sought to determine Infusor efficiency by measuring infusion lengths administered by Infusors to patients in the community setting and reviewing any impacting factors on varying infusion rates, if observed.
Method
Patients and nurses completed data collection forms daily over a 12-month period. The following information was recorded: time Infusor attached to patient, time Infusor emptied, Infusor ‘empty’ or ‘not empty’ when removed, volume of antibiotic solution remaining, Infusor storage details, antibiotic solution and dose, indication for treatment, and date (season). Statistical analyses was conducted using Stata. Data were analysed using descriptive statistics, including median and range for continuous variables, and frequency counts and percentages for categorical variables. Ethical approval was granted by Northern Sydney Local Health District (NSLHD) Research Office (Reference no: RESP/14/184), the Human Research Ethics Committee (HREC) (Reference no: LNR/14/HAWKE/265) and the study conforms to the Australian National Statement on Ethical Conduct in Human Research. Informed consent was obtained from all participants via a study information leaflet that was provided with the patient questionnaire and patients were informed that their participation in the study was optional. Patients indicated their consent by completing the data collection form for each day of treatment.
Results
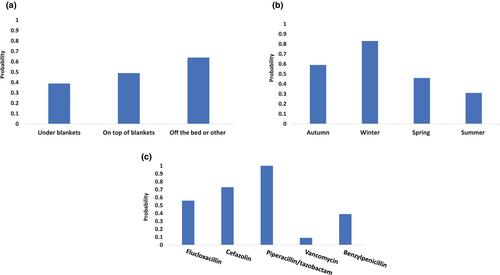
A significant number of Infusors (27%) emptied outside the expected infusion duration of 24 h ± 10% (21.6–26.4 h) and Infusors were removed ‘not empty’ when the nurse visited >24 h on 35% of occasions. Infusors were more likely to empty >24 h if they contained piperacillin-tazobactam 13.5 g (predicted probability = 1.0), in winter (predicted probability = 0.83), and in cooler overnight storage locations (predicted probability = 0.64). Infusors were more likely to empty <24 h if they contained vancomycin (predicted probability = 0.12).
Conclusion
Infusors delivering 24-h continuous intravenous infusions in the home setting may empty at unpredictable times and may be affected by temperature or solutions with varying doses. Outpatient parenteral antimicrobial therapy clinicians should be aware of possible unfinished infusions from Infusors.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


