Gut microbiome predicts cognitive function and depressive symptoms in late life
IF 10.1
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
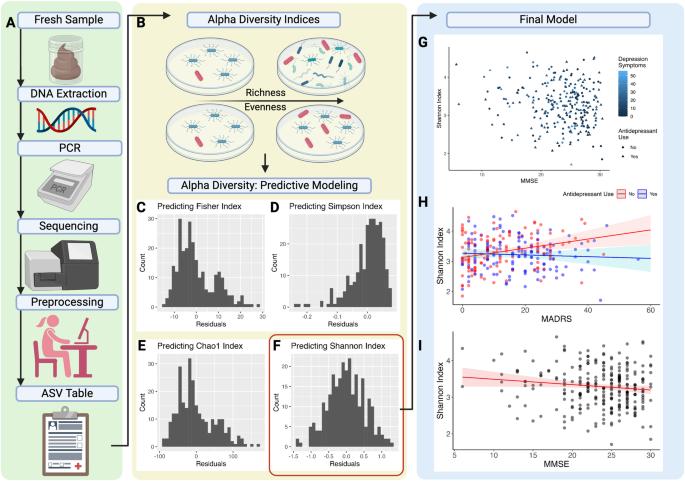
Depression in older adults with cognitive impairment increases progression to dementia. Microbiota is associated with current mood and cognition, but the extent to which it predicts future symptoms is unknown. In this work, we identified microbial features that reflect current and predict future cognitive and depressive symptoms. Clinical assessments and stool samples were collected from 268 participants with varying cognitive and depressive symptoms. Seventy participants underwent 2-year follow-up. Microbial community diversity, structure, and composition were assessed using high-resolution 16 S rRNA marker gene sequencing. We implemented linear regression to characterize the relationship between microbiome composition, current cognitive impairment, and depressive symptoms. We leveraged elastic net regression to discover features that reflect current or future cognitive function and depressive symptoms. Greater microbial community diversity associated with lower current cognition in the whole sample, and greater depression in participants not on antidepressants. Poor current cognitive function associated with lower relative abundance of Bifidobacterium, while greater GABA degradation associated with greater current depression severity. Future cognitive decline associated with lower cognitive function, lower relative abundance of Intestinibacter, lower glutamate degradation, and higher baseline histamine synthesis. Future increase in depressive symptoms associated with higher baseline depression and anxiety, lower cognitive function, diabetes, lower relative abundance of Bacteroidota, and lower glutamate degradation. Our results suggest cognitive dysfunction and depression are unique states with an overall biological effect detectable through gut microbiota. The microbiome may present a noninvasive readout and prognostic tool for cognitive and psychiatric states.
肠道微生物组预测晚年认知功能和抑郁症状
患有认知障碍的老年人中的抑郁症会加剧痴呆症的进展。微生物群与当前的情绪和认知能力有关,但其对未来症状的预测程度尚不清楚。在这项研究中,我们确定了能反映当前认知和抑郁症状并预测未来的微生物特征。我们收集了 268 名有不同认知和抑郁症状的参与者的临床评估结果和粪便样本。其中 70 人接受了为期 2 年的随访。我们使用高分辨率 16 S rRNA 标记基因测序对微生物群落的多样性、结构和组成进行了评估。我们采用线性回归法来描述微生物群组成、当前认知障碍和抑郁症状之间的关系。我们利用弹性网回归发现了反映当前或未来认知功能和抑郁症状的特征。在整个样本中,微生物群落多样性越高,当前认知功能越低,未服用抗抑郁药物的参与者抑郁程度越高。当前认知功能较差与双歧杆菌相对丰度较低有关,而 GABA 降解较多与当前抑郁严重程度较高有关。未来认知功能下降与认知功能较低、肠杆菌相对丰度较低、谷氨酸降解较低以及组胺合成基线较高有关。未来抑郁症状的增加与较高的基线抑郁和焦虑、较低的认知功能、糖尿病、较低的类杆菌相对丰度和较低的谷氨酸降解有关。我们的研究结果表明,认知功能障碍和抑郁是一种独特的状态,可通过肠道微生物群检测到整体生物效应。微生物组可能是认知和精神状态的非侵入性读数和预后工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Psychiatry
医学-精神病学
CiteScore
20.50
自引率
4.50%
发文量
459
审稿时长
4-8 weeks
期刊介绍:
Molecular Psychiatry focuses on publishing research that aims to uncover the biological mechanisms behind psychiatric disorders and their treatment. The journal emphasizes studies that bridge pre-clinical and clinical research, covering cellular, molecular, integrative, clinical, imaging, and psychopharmacology levels.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


