Stereochemistry matters: Inhibition of carbonic anhydrase II by amino acid derived sulfamates depends on their absolute configuration
European Journal of Medicinal Chemistry Reports
Pub Date : 2024-04-17
DOI:10.1016/j.ejmcr.2024.100162
引用次数: 0
Abstract
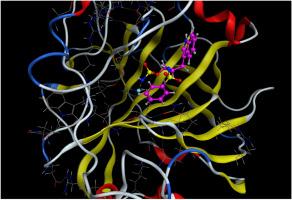
Aminoalcohols were converted into the corresponding enantiomeric phenylsulfonamide sulfamates. These compounds proved to be inhibitors of carbonic anhydrase II. Interestingly, a sulfamate derived from (S)-tryptophan was no inhibitor at all while its (R) configurated enantiomer was an excellent inhibitor of carbonic anhydrase II (CA II). A rationale can be deduced from molecular modeling studies. The sulfamates derived from (R) or (S) proline held very low inhibition constants for this enzyme as Ki = 0.77 μM and 0.70 μM, respectively.
立体化学很重要:氨基酸衍生的氨基磺酸盐对碳酸酐酶 II 的抑制作用取决于其绝对构型
氨基醇被转化为相应的对映体苯磺酰胺氨基磺酸盐。这些化合物被证明是碳酸酐酶 II 的抑制剂。有趣的是,从(S)-色氨酸中提取的氨基磺酸盐完全没有抑制作用,而其(R)构型对映体则是碳酸酐酶 II(CA II)的极佳抑制剂。从分子模型研究中可以推断出其中的道理。由(R)或(S)脯氨酸衍生的氨基磺酸盐对这种酶的抑制常数非常低,分别为 Ki = 0.77 μM 和 0.70 μM。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


