Nitrate promoted ATZ degradation in Z-scheme Cs3Bi2I9/BiVO4 photocatalytic system: Coupling ·OH and RNS mediated reduction and oxidation
引用次数: 0
Abstract
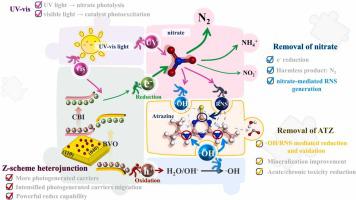
Nitrate, widely present in water, can be excited by UV light and generate reactive nitrogen species (RNS) etc., facilitating pollutants selective degradation. In this work, the promoting effect of nitrate on atrazine removal in the Z-scheme CsBiI/BiVO (CBI/BVO) photocatalytic system was studied. The CBI/BVO composite utilized CBI and BVO excitation to enhance photogenerated carriers production, and the Z-scheme heterojunction to increase photoinduced carriers separation yield, with a maximum value of 15.41 times that of pure BVO, releasing CBI electrons and BVO holes with powerful redox ability. Thanks to nitrate addition, the atrazine degradation improved, whose kinetic constant was 14.46 and 2.37 times higher than that of nitrate photolysis and 0.32-CBI/BVO photocatalysis. DFT, HPLC-MS, EPR, and toxicity assessments indicated the electron rich portion of atrazine and its intermediates were susceptible to RNS attack and underwent dechlorination, that combined with the oxidation brought by ·OH, synergistically promoting atrazine degradation, mineralization, and detoxification.
硝酸盐在 Z 型 Cs3Bi2I9/BiVO4 光催化体系中促进了 ATZ 的降解:耦合-OH和RNS介导的还原和氧化作用
硝酸盐广泛存在于水中,可被紫外光激发并产生活性氮物种(RNS)等,从而促进污染物的选择性降解。本文研究了硝酸盐对 Z 型 CsBiI/BiVO (CBI/BVO)光催化体系去除阿特拉津的促进作用。CBI/BVO复合材料利用CBI和BVO的激发提高了光生载流子的产生,Z-scheme异质结提高了光诱导载流子的分离产率,其最大值是纯BVO的15.41倍,释放出的CBI电子和BVO空穴具有强大的氧化还原能力。硝酸盐的加入改善了阿特拉津的降解,其动力学常数分别是硝酸盐光解和 0.32-CBI/BVO 光催化的 14.46 倍和 2.37 倍。DFT、HPLC-MS、EPR 和毒性评估表明,阿特拉津及其中间体的富电子部分易受 RNS 攻击并发生脱氯反应,再加上 -OH 带来的氧化作用,协同促进了阿特拉津的降解、矿化和解毒。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


