Panax notoginseng saponins prevent dementia and oxidative stress in brains of SAMP8 mice by enhancing mitophagy
IF 3.4
2区 医学
Q1 Medicine
引用次数: 0
Abstract
Mitochondrial dysfunction is one of the distinctive features of neurons in patients with Alzheimer’s disease (AD). Intraneuronal autophagosomes selectively phagocytose and degrade the damaged mitochondria, mitigating neuronal damage in AD. Panax notoginseng saponins (PNS) can effectively reduce oxidative stress and mitochondrial damage in the brain of animals with AD, but their exact mechanism of action is unknown. Senescence-accelerated mouse prone 8 (SAMP8) mice with age-related AD were treated with PNS for 8 weeks. The effects of PNS on learning and memory abilities, cerebral oxidative stress status, and hippocampus ultrastructure of mice were observed. Moreover, changes of the PTEN-induced putative kinase 1 (PINK1)-Parkin, which regulates ubiquitin-dependent mitophagy, and the recruit of downstream autophagy receptors were investigated. PNS attenuated cognitive dysfunction in SAMP8 mice in the Morris water maze test. PNS also enhanced glutathione peroxidase and superoxide dismutase activities, and increased glutathione levels by 25.92% and 45.55% while inhibiting 8-hydroxydeoxyguanosine by 27.74% and the malondialdehyde production by 34.02% in the brains of SAMP8 mice. Our observation revealed the promotion of mitophagy, which was accompanied by an increase in microtubule-associated protein 1 light chain 3 (LC3) mRNA and 70.00% increase of LC3-II/I protein ratio in the brain tissues of PNS-treated mice. PNS treatment increased Parkin mRNA and protein expression by 62.80% and 43.80%, while increasing the mRNA transcription and protein expression of mitophagic receptors such as optineurin, and nuclear dot protein 52. PNS enhanced the PINK1/Parkin pathway and facilitated mitophagy in the hippocampus, thereby preventing cerebral oxidative stress in SAMP8 mice. This may be a mechanism contributing to the cognition-improvement effect of PNS.
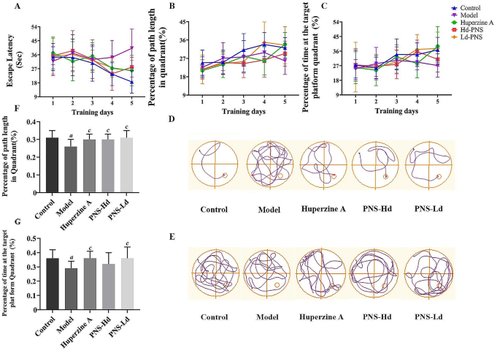
三七皂苷通过增强有丝分裂防止 SAMP8 小鼠大脑痴呆和氧化应激
线粒体功能障碍是阿尔茨海默病患者神经元的显著特征之一。神经元内的自噬体可选择性地吞噬和降解受损的线粒体,减轻阿尔茨海默病对神经元的损伤。三七皂苷(PNS)能有效减轻AD动物大脑中的氧化应激和线粒体损伤,但其确切的作用机制尚不清楚。研究人员用 PNS 对患有老年性注意力缺失症的衰老加速小鼠易感基因 8(SAMP8)小鼠进行了为期 8 周的治疗。观察了 PNS 对小鼠学习和记忆能力、大脑氧化应激状态和海马超微结构的影响。此外,还研究了调控泛素依赖性有丝分裂的PTEN诱导的推定激酶1(PINK1)-Parkin的变化以及下游自噬受体的招募情况。在莫里斯水迷宫测试中,PNS 可减轻 SAMP8 小鼠的认知功能障碍。PNS 还提高了谷胱甘肽过氧化物酶和超氧化物歧化酶的活性,谷胱甘肽水平分别提高了 25.92% 和 45.55%,同时抑制了 8-羟基脱氧鸟苷 27.74% 和丙二醛产生 34.02%。我们的观察结果表明,PNS 治疗小鼠脑组织中的微管相关蛋白 1 轻链 3(LC3)mRNA 增加,LC3-II/I 蛋白比值增加 70.00%,从而促进了有丝分裂。PNS 治疗可使 Parkin mRNA 和蛋白表达量分别增加 62.80% 和 43.80%,同时还可增加有丝分裂受体(如 optineurin)和核点蛋白 52 的 mRNA 转录量和蛋白表达量。PNS 增强了 PINK1/Parkin 通路,促进了海马中的有丝分裂,从而防止了 SAMP8 小鼠的脑氧化应激。这可能是 PNS 改善认知效果的一个机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

BMC Complementary and Alternative Medicine
INTEGRATIVE & COMPLEMENTARY MEDICINE-
CiteScore
7.00
自引率
0.00%
发文量
0
审稿时长
3 months
期刊介绍:
BMC Complementary Medicine and Therapies is an open access journal publishing original peer-reviewed research articles on interventions and resources that complement or replace conventional therapies, with a specific emphasis on research that explores the biological mechanisms of action, as well as their efficacy, safety, costs, patterns of use and/or implementation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


