Yanjiang Chen, Sabrina Steiner, Catherine Hagedorn, Sarah Kollar, Alicia Pliego-Mendieta, Martina Haberecker, Jan Plock, Christian Britschgi, Lara Planas-Paz, Chantal Pauli
下载PDF
{"title":"Acquired NF2 mutation confers resistance to TRK inhibition in an ex vivo LMNA::NTRK1-rearranged soft-tissue sarcoma cell model","authors":"Yanjiang Chen, Sabrina Steiner, Catherine Hagedorn, Sarah Kollar, Alicia Pliego-Mendieta, Martina Haberecker, Jan Plock, Christian Britschgi, Lara Planas-Paz, Chantal Pauli","doi":"10.1002/path.6282","DOIUrl":null,"url":null,"abstract":"<p>Genomic rearrangements of the neurotrophic receptor tyrosine kinase genes (<i>NTRK1, NTRK2</i>, and <i>NTRK3</i>) are the most common mechanism of oncogenic activation for this family of receptors, resulting in sustained cancer cell proliferation. Several targeted therapies have been approved for tumours harbouring <i>NTRK</i> fusions and a new generation of TRK inhibitors has already been developed due to acquired resistance. We established a patient-derived <i>LMNA</i>::<i>NTRK1</i>-rearranged soft-tissue sarcoma cell model <i>ex vivo</i> with an acquired resistance to targeted TRK inhibition. Molecular profiling of the resistant clones revealed an acquired <i>NF2</i> loss of function mutation that was absent in the parental cell model. Parental cells showed continuous sensitivity to TRK-targeted treatment, whereas the resistant clones were insensitive. Furthermore, resistant clones showed upregulation of the MAPK and mTOR/AKT pathways in the gene expression based on RNA sequencing data and increased sensitivity to MEK and mTOR inhibitor therapy. Drug synergy was seen using trametinib and rapamycin in combination with entrectinib. Medium-throughput drug screening further identified small compounds as potential drug candidates to overcome resistance as monotherapy or in combination with entrectinib. In summary, we developed a comprehensive model of drug resistance in an <i>LMNA</i>::<i>NTRK1</i>-rearranged soft-tissue sarcoma and have broadened the understanding of acquired drug resistance to targeted TRK therapy. Furthermore, we identified drug combinations and small compounds to overcome acquired drug resistance and potentially guide patient care in a functional precision oncology setting. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 2","pages":"257-269"},"PeriodicalIF":5.6000,"publicationDate":"2024-04-12","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6282","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6282","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
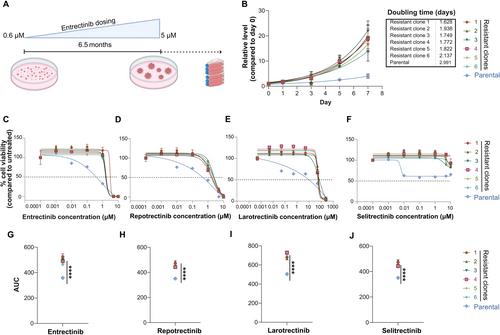
Genomic rearrangements of the neurotrophic receptor tyrosine kinase genes (NTRK1, NTRK2 , and NTRK3 ) are the most common mechanism of oncogenic activation for this family of receptors, resulting in sustained cancer cell proliferation. Several targeted therapies have been approved for tumours harbouring NTRK fusions and a new generation of TRK inhibitors has already been developed due to acquired resistance. We established a patient-derived LMNA ::NTRK1 -rearranged soft-tissue sarcoma cell model ex vivo with an acquired resistance to targeted TRK inhibition. Molecular profiling of the resistant clones revealed an acquired NF2 loss of function mutation that was absent in the parental cell model. Parental cells showed continuous sensitivity to TRK-targeted treatment, whereas the resistant clones were insensitive. Furthermore, resistant clones showed upregulation of the MAPK and mTOR/AKT pathways in the gene expression based on RNA sequencing data and increased sensitivity to MEK and mTOR inhibitor therapy. Drug synergy was seen using trametinib and rapamycin in combination with entrectinib. Medium-throughput drug screening further identified small compounds as potential drug candidates to overcome resistance as monotherapy or in combination with entrectinib. In summary, we developed a comprehensive model of drug resistance in an LMNA ::NTRK1 -rearranged soft-tissue sarcoma and have broadened the understanding of acquired drug resistance to targeted TRK therapy. Furthermore, we identified drug combinations and small compounds to overcome acquired drug resistance and potentially guide patient care in a functional precision oncology setting. © 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
在体外LMNA::NTRK1重排软组织肉瘤细胞模型中,获得性NF2突变使细胞对TRK抑制剂产生抗药性
神经营养受体酪氨酸激酶基因(NTRK1、NTRK2 和 NTRK3)的基因组重排是该受体家族最常见的致癌激活机制,可导致癌细胞持续增殖。针对携带NTRK融合的肿瘤,已有几种靶向疗法获得批准,而由于获得性免疫耐药,新一代TRK抑制剂也已开发出来。我们在体外建立了一个源自患者的LMNA::NTRK1重组软组织肉瘤细胞模型,该模型对靶向TRK抑制剂具有获得性耐药性。耐药克隆的分子图谱显示,亲代细胞模型中不存在获得性 NF2 功能缺失突变。亲代细胞对 TRK 靶向治疗表现出持续的敏感性,而耐药克隆则不敏感。此外,根据 RNA 测序数据,耐药克隆的 MAPK 和 mTOR/AKT 通路基因表达上调,对 MEK 和 mTOR 抑制剂治疗的敏感性增加。曲美替尼和雷帕霉素与恩曲替尼联合使用可产生药物协同作用。中通量药物筛选进一步确定了一些小化合物,作为单药治疗或与恩替替尼联合治疗克服耐药性的潜在候选药物。总之,我们建立了一个全面的LMNA::NTRK1重组软组织肉瘤耐药模型,拓宽了人们对TRK靶向治疗获得性耐药性的认识。此外,我们还确定了克服获得性耐药性的药物组合和小化合物,并有可能在功能性精准肿瘤学环境中指导患者治疗。© 2024 作者。病理学杂志》由 John Wiley & Sons Ltd 代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


