Alpha-1 adrenergic antagonists and the risk of hospitalization or death in non-hospitalized patients with COVID-19: A population-based study
Abstract
Background
Alpha-1 receptor antagonists may interfere with IL-6 signaling and could therefore be a potential treatment for COVID-19. However, the effectiveness of these drugs in mitigating the risk of clinical deterioration among non-hospitalized patients with COVID-19 is unknown.
Objectives
The aim of this study is to examine the association between alpha-1 antagonist exposure and the 30-day risk of a hospital encounter or death in nonhospitalized patients with COVID-19.
Methods
We conducted a population-based cohort study of Ontario residents aged 35 years and older who were eligible for public drug coverage and who had a positive test for SARS-CoV-2 between January 1, 2020, and March 1, 2021. We matched each individual receiving an alpha-1 antagonist at the time of their positive test with two non-exposed individuals using propensity scores. Our outcome was a composite of a hospital admission, emergency department visit, or death, 1 to 30 days following the positive test.
Results
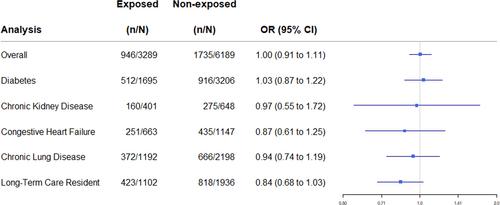
We matched 3289 alpha-1 antagonist exposed patients to 6189 unexposed patients. Overall, there was no difference in the 30-day risk of the primary outcome among patients exposed to alpha-1 antagonists at the time of their diagnosis relative to unexposed individuals (28.8% vs. 28.0%; OR 1.00, 95% CI 0.91 to 1.11). In a secondary analysis, individuals exposed to alpha-1 antagonists had a lower risk of death in the 30 days following a COVID diagnosis (OR 0.79; 95% CI 0.66 to 0.93).
Conclusion
Alpha-1 antagonists did not mitigate the 30-day risk of clinical deterioration in non-hospitalized patients with COVID-19. Our findings do not support the general repurposing of alpha-1 antagonists as a treatment for such patients, although there may be subgroups of patients in whom further research is warranted.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


