Effect of pre‐emptive rituximab on EBV DNA levels and prevention of post‐transplant lymphoproliferative disorder in pediatric kidney transplant recipients: A case series from the pediatric nephrology research consortium
IF 1.2
4区 医学
Q3 PEDIATRICS
引用次数: 0
Abstract
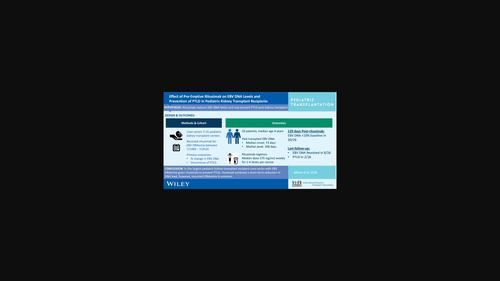
BackgroundThere are scant data on the effect of rituximab on EBV DNA levels and prevention of post‐transplant lymphoproliferative disorder (PTLD) in pediatric kidney transplant recipients with EBV DNAemia.MethodsKidney transplant recipients with EBV DNAemia treated with rituximab to prevent PTLD between 7/1999 and 7/2019 at five pediatric centers were included. Those with confirmed PTLD at the onset of rituximab were excluded. Primary outcomes included percentage change in EBV DNAemia and occurrence of PTLD post rituximab.ResultsTwenty‐six pediatric kidney transplant recipients were included. Median age at transplant was 4 years (IQR 2.1–10.3). EBV DNA load monitoring by qPCR was performed at 1–3 month intervals. EBV DNAemia onset occurred at a median of 73 days post‐transplant (IQR 52–307), followed by DNAemia peak at a median of 268 days (IQR 112–536). Rituximab was administered at a median of 9 days post peak (IQR 0–118). Rituximab regimens varied; median dose 375 mg/m
抢先使用利妥昔单抗对小儿肾移植受者体内 EBV DNA 水平的影响以及对移植后淋巴组织增生性疾病的预防:儿科肾脏病研究联盟的病例系列
背景关于利妥昔单抗对EBV DNA水平的影响以及预防患有EBV DNA血症的小儿肾移植受者移植后淋巴组织增生性疾病(PTLD)的数据很少。排除那些在开始使用利妥昔单抗时已确诊患有PTLD的受者。主要结果包括EBV DNA血症的百分比变化和利妥昔单抗后PTLD的发生率。中位移植年龄为 4 岁(IQR 2.1-10.3)。每隔 1-3 个月通过 qPCR 监测一次 EBV DNA 负载。EBV DNA血症发病时间中位数为移植后 73 天(IQR 52-307),DNA血症高峰期中位数为 268 天(IQR 112-536)。利妥昔单抗用药时间中位数为高峰后 9 天(IQR 0-118)。利妥昔单抗方案各不相同;中位剂量为 375 毫克/平方米(IQR 375-439),每周一次,每个疗程 1-4 次。使用利妥昔单抗后,20/26 例患者的 EBV DNA 负荷在 120 天时降至基线的 10%;然而,只有 30% 的患者在最后一次随访时(移植后中位数 2094 天 [IQR 1538-3463])达到完全清除。两名患者(7%)在利妥昔单抗应用后 915 天和 1713 天出现 PTLD。所有受者的移植物均正常。结论 在规模最大的儿科肾移植受者病例系列中,EBV DNA血症患者使用利妥昔单抗预防PTLD,利妥昔单抗可在短期内减少DNA载量;但复发性DNA血症很常见。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pediatric Transplantation
医学-小儿科
CiteScore
2.90
自引率
15.40%
发文量
216
审稿时长
3-8 weeks
期刊介绍:
The aim of Pediatric Transplantation is to publish original articles of the highest quality on clinical experience and basic research in transplantation of tissues and solid organs in infants, children and adolescents. The journal seeks to disseminate the latest information widely to all individuals involved in kidney, liver, heart, lung, intestine and stem cell (bone-marrow) transplantation. In addition, the journal publishes focused reviews on topics relevant to pediatric transplantation as well as timely editorial comment on controversial issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


