Predicting stroke risk after sepsis hospitalization with new-onset atrial fibrillation
Abstract
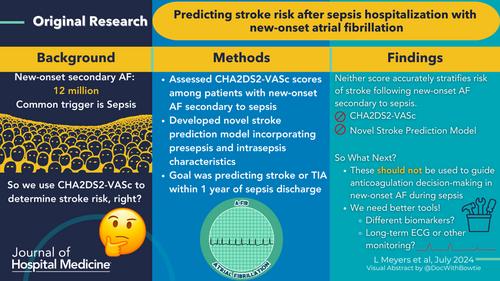
Background
New-onset atrial fibrillation (AF) during sepsis is common, but models designed to stratify stroke risk excluded patients with secondary AF. We assessed the predictive validity of CHA2DS2VASc scores among patients with new-onset AF during sepsis and developed a novel stroke prediction model incorporating presepsis and intrasepsis characteristics.
Methods
We included patients ≥40 years old who survived hospitalizations with sepsis and new-onset AF across 21 Kaiser Permanente Northern California hospitals from January 1, 2011 to September 30, 2017. We calculated the area under the receiver operating curve (AUC) for CHA2DS2VASc scores to predict stroke or transient ischemic attack (TIA) within 1 year after a hospitalization with new-onset AF during sepsis using Fine-Gray models with death as competing risk. We similarly derived and validated a novel model using presepsis and intrasepsis characteristics associated with 1-year stroke/TIA risk.
Results
Among 82,748 adults hospitalized with sepsis, 3992 with new-onset AF (median age: 80 years, median CHA2DS2VASc of 4) survived to discharge, among whom 70 (2.1%) experienced stroke or TIA outcome and 1393 (41.0%) died within 1 year of sepsis. The CHA2DS2VASc score was not predictive of stroke risk after sepsis (AUC: 0.50, 95% confidence interval [CI]: 0.48–0.52). A newly derived model among 2555 (64%) patients in the derivation set and 1437 (36%) in the validation set included 13 variables and produced an AUC of 0.61 (0.49–0.73) in derivation and 0.54 (0.43–0.65) in validation.
Conclusion
Current models do not accurately stratify risk of stroke following new-onset AF secondary to sepsis. New tools are required to guide anticoagulation decisions following new-onset AF in sepsis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


