A Kinetic Model of Catalytic Homocondensation of Acetone into Mesitylene
IF 1.3
4区 工程技术
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
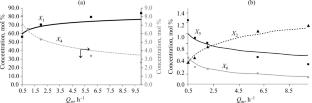
The catalytic properties of mesoporous amorphous titanosilicate Ti–Si in acetone homocondensation at 250–350°С and mass feed space velocity of 0.5–10 h–1 were studied. The acetone conversion was 13–52 wt %, and the selectivity with respect to mesitylene was 52–70 wt %. A kinetic model based on the experimentally developed scheme of chemical transformations was constructed; it includes 10 steps and takes into account the effect of water. The retrieval of the kinetic parameters was implemented in the form of a global optimization problem, which allowed determination of the kinetic constants, adsorption constant, activation energy, and heat of adsorption of water.
丙酮催化均缩为甲撑苯的动力学模型
摘要 研究了介孔无定形钛硅酸盐 Ti-Si 在 250-350°С 和 0.5-10 h-1 质量进料空间速度条件下丙酮均缩反应的催化特性。丙酮的转化率为 13-52 wt %,对间苯的选择性为 52-70 wt %。根据实验开发的化学转化方案构建了一个动力学模型;该模型包括 10 个步骤,并考虑了水的影响。动力学参数的检索以全局优化问题的形式进行,从而确定了动力学常数、吸附常数、活化能和水的吸附热。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Petroleum Chemistry
工程技术-工程:化工
CiteScore
2.50
自引率
21.40%
发文量
102
审稿时长
6-12 weeks
期刊介绍:
Petroleum Chemistry (Neftekhimiya), founded in 1961, offers original papers on and reviews of theoretical and experimental studies concerned with current problems of petroleum chemistry and processing such as chemical composition of crude oils and natural gas liquids; petroleum refining (cracking, hydrocracking, and catalytic reforming); catalysts for petrochemical processes (hydrogenation, isomerization, oxidation, hydroformylation, etc.); activation and catalytic transformation of hydrocarbons and other components of petroleum, natural gas, and other complex organic mixtures; new petrochemicals including lubricants and additives; environmental problems; and information on scientific meetings relevant to these areas.
Petroleum Chemistry publishes articles on these topics from members of the scientific community of the former Soviet Union.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


