Dose-response evaluation of intravenous gene therapy in a symptomatic mouse model of metachromatic leukodystrophy
IF 4.6
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Molecular Therapy-Methods & Clinical Development
Pub Date : 2024-04-06
DOI:10.1016/j.omtm.2024.101248
引用次数: 0
Abstract
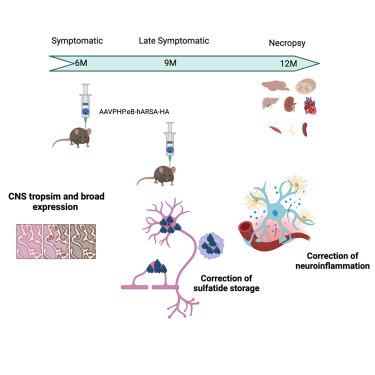
Metachromatic leukodystrophy (MLD) is a rare, autosomal recessive neurodegenerative disease caused by deficient activity of the lysosomal enzyme arylsulfatase A (ARSA), resulting in sulfatide accumulation and subsequent demyelination and neuronal damage within the central and peripheral nervous systems. Three clinical forms of MLD have been described, based on age at symptom onset. The most frequent and severe forms have an early onset, with the disease progressing rapidly toward severe motor and cognitive regression and ultimately premature death. There are currently no approved therapies for most of these early-onset patients once symptoms are present. Thus, it is crucial to develop new approaches to treat symptomatic patients. Here, we proposed a gene therapy approach based on the intravenous delivery of AAVPHP.eB encoding ARSA. MLD mice were treated at 6 months for a dose-response study and at 9 months to assess late-treatment efficacy. Therapeutic efficacy was evaluated 3 or 6 months after injection. We demonstrated a broad transduction in the central nervous system, a complete correction of sulfatide storage, and a significant improvement in neuroinflammation at low dose and late treatment. Taken together, this work establishes a strong rationale for proposing a phase I/II clinical trial in MLD patients.
变色性白质营养不良症无症状小鼠模型中静脉注射基因疗法的剂量-反应评估
变色性白质营养不良症(MLD)是一种罕见的常染色体隐性神经退行性疾病,其病因是溶酶体酶 Arylsulfatase A(ARSA)活性不足,导致硫化物蓄积,进而引起中枢神经系统和周围神经系统脱髓鞘和神经元损伤。根据症状出现时的年龄,已描述了三种临床形式的 MLD。最常见和最严重的形式起病较早,病情发展迅速,运动和认知能力严重退化,最终导致过早死亡。目前,大多数早发患者一旦出现症状,还没有获得批准的治疗方法。因此,开发治疗无症状患者的新方法至关重要。在此,我们提出了一种基于静脉注射编码 ARSA 的 AAVPHP.eB 的基因治疗方法。MLD 小鼠在 6 个月时接受治疗以进行剂量反应研究,在 9 个月时接受治疗以评估后期疗效。治疗效果在注射后 3 个月或 6 个月进行评估。我们证明了低剂量和晚期治疗对中枢神经系统的广泛转导、硫化物储存的完全纠正以及神经炎症的显著改善。综上所述,这项研究为在 MLD 患者中开展 I/II 期临床试验提供了强有力的依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Therapy-Methods & Clinical Development
Biochemistry, Genetics and Molecular Biology-Molecular Biology
CiteScore
9.90
自引率
4.30%
发文量
163
审稿时长
12 weeks
期刊介绍:
The aim of Molecular Therapy—Methods & Clinical Development is to build upon the success of Molecular Therapy in publishing important peer-reviewed methods and procedures, as well as translational advances in the broad array of fields under the molecular therapy umbrella.
Topics of particular interest within the journal''s scope include:
Gene vector engineering and production,
Methods for targeted genome editing and engineering,
Methods and technology development for cell reprogramming and directed differentiation of pluripotent cells,
Methods for gene and cell vector delivery,
Development of biomaterials and nanoparticles for applications in gene and cell therapy and regenerative medicine,
Analysis of gene and cell vector biodistribution and tracking,
Pharmacology/toxicology studies of new and next-generation vectors,
Methods for cell isolation, engineering, culture, expansion, and transplantation,
Cell processing, storage, and banking for therapeutic application,
Preclinical and QC/QA assay development,
Translational and clinical scale-up and Good Manufacturing procedures and process development,
Clinical protocol development,
Computational and bioinformatic methods for analysis, modeling, or visualization of biological data,
Negotiating the regulatory approval process and obtaining such approval for clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


