Designer tryptophan-rich peptide modulates structural dynamics of HIF-1α DNA i-motif DNA
IF 1.8
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cytosine-rich DNA sequences can fold into intercalated motifs known as i-motifs, through noncanonical hydrogen bonding interactions. Molecular probes can provide valuable insights into the conformational stability and potential cellular functions of i-motifs. W5K5, a decapeptide composed of alternating tryptophan (W) and lysine (K) units, has been identified as a lead candidate to modulate the structural dynamics of the hypoxia-inducible factor 1-alpha (HIF-1α) DNA i-motif. This finding is expected to facilitate the rational design of peptide-based probes for studying the structure and functional dynamics of i-motifs.
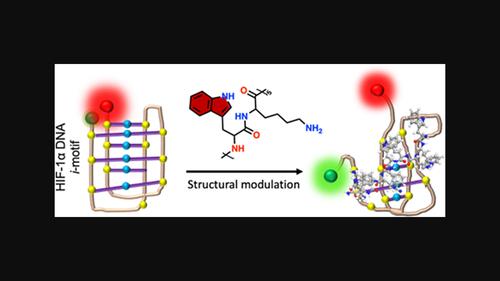
富含色氨酸的设计肽调节 HIF-1α DNA i-motif DNA 的结构动态
富含胞嘧啶的 DNA 序列可以通过非经典氢键相互作用折叠成被称为 i-motifs 的插层图案。分子探针可以为了解 i-motifs的构象稳定性和潜在的细胞功能提供宝贵的信息。W5K5是一种由色氨酸(W)和赖氨酸(K)单位交替组成的十肽,已被确定为调节缺氧诱导因子1-α(HIF-1α)DNA i-motif结构动态的主要候选物。这一发现有望促进基于多肽的探针的合理设计,以研究i-motifs的结构和功能动态。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Peptide Science
生物-分析化学
CiteScore
3.40
自引率
4.80%
发文量
83
审稿时长
1.7 months
期刊介绍:
The official Journal of the European Peptide Society EPS
The Journal of Peptide Science is a cooperative venture of John Wiley & Sons, Ltd and the European Peptide Society, undertaken for the advancement of international peptide science by the publication of original research results and reviews. The Journal of Peptide Science publishes three types of articles: Research Articles, Rapid Communications and Reviews.
The scope of the Journal embraces the whole range of peptide chemistry and biology: the isolation, characterisation, synthesis properties (chemical, physical, conformational, pharmacological, endocrine and immunological) and applications of natural peptides; studies of their analogues, including peptidomimetics; peptide antibiotics and other peptide-derived complex natural products; peptide and peptide-related drug design and development; peptide materials and nanomaterials science; combinatorial peptide research; the chemical synthesis of proteins; and methodological advances in all these areas. The spectrum of interests is well illustrated by the published proceedings of the regular international Symposia of the European, American, Japanese, Australian, Chinese and Indian Peptide Societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


