The reaction of 4-hydroxy-6H-1,3-oxazin-6-ones with amidines – a route to access new 1,3,5-triazine derivatives
IF 1
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The reaction of 2-aryl-5-methyl-4-hydroxy-6H-1,3-oxazin-6-ones with 1,3-binucleophilic reagents acetamidine and benzamidine was studied. It was established that in n-propanol under reflux in the presence of sodium n-propoxide or in DMSO, the predominant reaction products were 1,3,5-triazine derivatives. It was shown that the reaction time and the yield of the target product were significantly influenced by the choice of the solvent, the nucleophilicity of the amidine, and the electronic structure of 4 hydroxy-6H-1,3-oxazin-6-one.
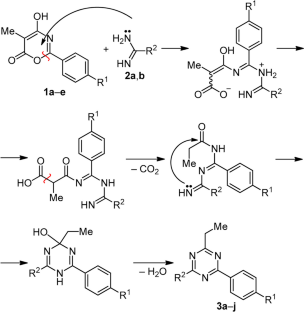
4-羟基-6H-1,3-恶嗪-6-酮与酰胺的反应--获得新的 1,3,5-三嗪衍生物的途径
研究了 2-芳基-5-甲基-4-羟基-6H-1,3-恶嗪-6-酮与 1,3-亲核试剂乙脒和联苯胺的反应。结果表明,在正丙醇中,在正丙氧基钠存在下回流或在二甲基亚砜中,主要的反应产物是 1,3,5-三嗪衍生物。研究表明,溶剂的选择、脒的亲核性和 4-羟基-6H-1,3-恶嗪-6-酮的电子结构对反应时间和目标产物的产率有显著影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
2.90
自引率
13.30%
发文量
98
审稿时长
1 months
期刊介绍:
The international journal Chemistry of Heterocyclic Compounds publishes original papers, short communications, reviews, and mini-reviews dealing with problems in the field of heterocyclic chemistry in Russian and English. The Journal also publishes reviews and annotations on new books and brief reports on conferences in the field of heterocyclic chemistry, as well as commemoratives dedicated to prominent heterocyclic chemists.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


