Metabolite profiling of foslevodopa/foscarbidopa in plasma of healthy human participants by LC‐HRMS indicates no major differences compared to administration of levodopa/carbidopa intestinal gel
IF 2.9
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
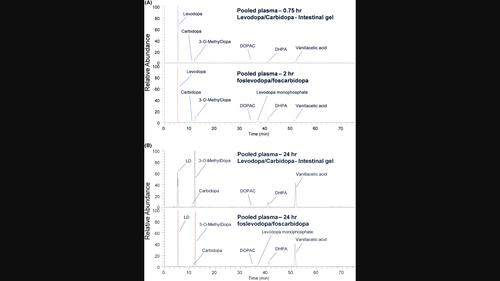
Analysis was conducted to compare levodopa/carbidopa pharmacokinetics and drug‐related material in plasma of healthy participants after receiving a continuous infusion of Levodopa/Carbidopa Intestinal Gel (LCIG) to a continuous subcutaneous infusion of foslevodopa/foscarbidopa. Study samples were from a randomized, open‐label, 2‐period crossover study in 20 healthy participants. Participants received either 24‐h foslevodopa/foscarbidopa SC infusion to the abdomen or LCIG delivered for 24 h to the jejunum through a nasogastric tube with jejunal extension. Serial blood samples were collected for PK. Comparability of the LD PK parameters between the two treatment regimens was determined. Selected plasma samples were pooled per treatment group and per time point for metabolite profiling. LC–MS
通过 LC-HRMS 对健康人血浆中的磷左旋多巴/磷卡比多巴进行代谢物分析表明,与服用左旋多巴/卡比多巴肠道凝胶相比没有重大差异
本研究对健康参与者在连续输注左旋多巴/卡比多巴肠溶凝胶(LCIG)和连续皮下注射磷左旋多巴/磷卡比多巴后血浆中左旋多巴/卡比多巴的药代动力学和药物相关物质进行了分析比较。研究样本来自一项随机、开放标签、2 期交叉研究,共有 20 名健康参与者参加。研究人员在腹部接受了 24 小时的福斯莱多巴/福斯卡比多巴皮下注射,或通过带有空肠延伸的鼻胃管在空肠接受了 24 小时的低密度脂蛋白胆碱酯酶(LCIG)注射。采集连续血样进行 PK 分析。确定两种治疗方案的 LD PK 参数是否具有可比性。每个治疗组和每个时间点的部分血浆样本被集中起来进行代谢物分析。使用高分辨率质谱进行 LC-MSn 分析,以确定不同给药方案和时间点的药物相关物质。相对于LCIG输注,福沙窝多巴/福斯卡比多巴皮下注射后的LD PK参数中心值和90%置信区间介于0.8和1.25之间。给予 LCIG 后,在早期和晚期时间点(0.75 小时和 24 小时)的血浆中发现了 LD、CD、3-OMD、DHPA、DOPAC 和香草酸;给予磷左多巴/磷卡比多巴后的代谢轮廓显示出相同的药物相关化合物,但给予的磷左多巴除外。在两种治疗方案中,3-OMD 和香草酸的含量均随时间推移而增加。液相色谱-质谱峰面积的相对定量显示,代谢物的分布没有重大差异。这些结果表明,与 LCIG 相比,添加单磷酸原药分子或 SC 给药均不会影响福斯雷多巴/福斯卡比多巴的循环代谢物谱。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacology Research & Perspectives
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
5.30
自引率
3.80%
发文量
120
审稿时长
20 weeks
期刊介绍:
PR&P is jointly published by the American Society for Pharmacology and Experimental Therapeutics (ASPET), the British Pharmacological Society (BPS), and Wiley. PR&P is a bi-monthly open access journal that publishes a range of article types, including: target validation (preclinical papers that show a hypothesis is incorrect or papers on drugs that have failed in early clinical development); drug discovery reviews (strategy, hypotheses, and data resulting in a successful therapeutic drug); frontiers in translational medicine (drug and target validation for an unmet therapeutic need); pharmacological hypotheses (reviews that are oriented to inform a novel hypothesis); and replication studies (work that refutes key findings [failed replication] and work that validates key findings). PR&P publishes papers submitted directly to the journal and those referred from the journals of ASPET and the BPS
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


