Absorption and pharmacokinetics of bupivacaine after bilateral topical administration in tonsillar fossae for posttonsillectomy pain relief
IF 2.9
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
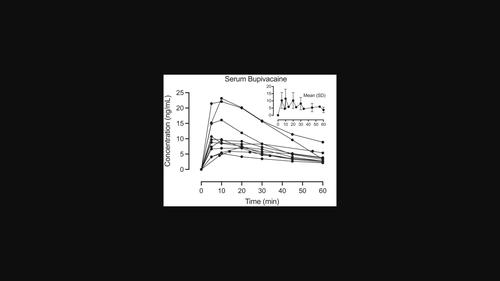
No previous studies have investigated the systemic absorption of bupivacaine when used topically for posttonsillectomy pain. The present study was undertaken to investigate the pharmacokinetics of bupivacaine after administration by a swab in the tonsillar fossae over 4 min after tonsillectomy. Eleven adult patients undergoing elective tonsillectomy were recruited. After removal of both tonsils, each of the two tonsillar fossae was covered with a swab moistened with 2 mL of bupivacaine 5 mg/mL, that is, a total of 20 mg bupivacaine. Blood samples were drawn after 0, 5, 10, 20, 30, 45, and 60 min. Bupivacaine was analyzed with an ultra‐high‐performance liquid chromatography–tandem mass spectrometry method. The highest single measured bupivacaine serum concentration was 23.2 ng/mL and took place 10 min after drug administration. Mean (±SD) C
扁桃体切除术后疼痛缓解双侧扁桃体窝局部给药后布比卡因的吸收和药代动力学
以前没有研究调查过局部使用布比卡因治疗扁桃体切除术后疼痛时的全身吸收情况。本研究旨在探讨扁桃体切除术后 4 分钟内用拭子在扁桃体窝给药后布比卡因的药代动力学。本研究招募了 11 名接受扁桃体切除术的成年患者。切除双侧扁桃体后,在两侧扁桃体窝处各涂上 2 毫升 5 毫克/毫升布比卡因(即总共 20 毫克布比卡因)的拭子。分别在 0、5、10、20、30、45 和 60 分钟后抽取血液样本。布比卡因采用超高效液相色谱-串联质谱法进行分析。布比卡因的单次最高血清浓度为 23.2 纳克/毫升,出现在用药后 10 分钟。平均(±SD)Cmax 为 11.4 ± 6.0 纳克/毫升,平均 tmax 为 11.3 ± 4.7 分钟。平均 t1/2 为 31.6 ± 9.3 分钟。据报道,毒性浓度阈值介于 1500-4500 纳克/毫升之间,因此测得的浓度远低于最低毒性阈值的 2%。总之,这项研究表明,在扁桃体切除术后的扁桃体窝中用拭子涂抹 4 毫升 5 毫克/毫升的布比卡因可产生极低的血浆浓度,这表明布比卡因的应用是安全的,没有任何全身毒性反应的风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacology Research & Perspectives
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
5.30
自引率
3.80%
发文量
120
审稿时长
20 weeks
期刊介绍:
PR&P is jointly published by the American Society for Pharmacology and Experimental Therapeutics (ASPET), the British Pharmacological Society (BPS), and Wiley. PR&P is a bi-monthly open access journal that publishes a range of article types, including: target validation (preclinical papers that show a hypothesis is incorrect or papers on drugs that have failed in early clinical development); drug discovery reviews (strategy, hypotheses, and data resulting in a successful therapeutic drug); frontiers in translational medicine (drug and target validation for an unmet therapeutic need); pharmacological hypotheses (reviews that are oriented to inform a novel hypothesis); and replication studies (work that refutes key findings [failed replication] and work that validates key findings). PR&P publishes papers submitted directly to the journal and those referred from the journals of ASPET and the BPS
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


