Ursodeoxycholic acid may protect from severe acute respiratory syndrome coronavirus 2 Omicron variant by reducing angiotensin‐converting enzyme 2
IF 2.9
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The SARS‐CoV‐2 caused COVID‐19 pandemic has posed a global health hazard. While some vaccines have been developed, protection against viral infection is not perfect because of the urgent approval process and the emergence of mutant SARS‐CoV‐2 variants. Here, we employed UDCA as an FXR antagonist to regulate ACE2 expression, which is one of the key pathways activated by SARS‐CoV‐2 Delta variant infection. UDCA is a well‐known reagent of liver health supplements and the only clinically approved bile acid. In this paper, we investigated the protective efficacy of UDCA on Omicron variation, since it has previously been verified for protection against Delta variant. When co‐housing with an Omicron variant‐infected hamster group resulted in spontaneous airborne transmission, the UDCA pre‐supplied group was protected from weight loss relative to the non‐treated group at 4 days post‐infection by more than 5%–10%. Furthermore, UDCA‐treated groups had a 3‐fold decrease in ACE2 expression in nasal cavities, as well as reduced viral expressing genes in the respiratory tract. Here, the data show that the UDCA serves an alternative option for preventive drug, providing SARS‐CoV‐2 protection against not only Delta but also Omicron variant. Our results of this study will help to propose drug‐repositioning of UDCA from liver health supplement to preventive drug of SARS‐CoV‐2 infection.
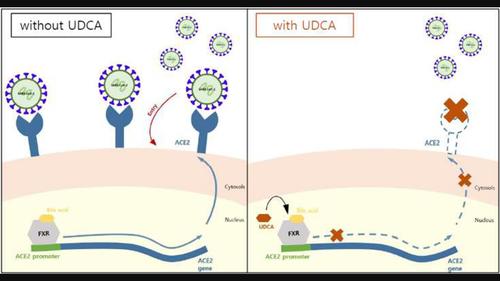
熊去氧胆酸可通过减少血管紧张素转换酶 2 保护人体免受严重急性呼吸系统综合征冠状病毒 2 Omicron 变体的感染
由 SARS-CoV-2 引起的 COVID-19 大流行已对全球健康造成危害。虽然已经开发出了一些疫苗,但由于审批程序紧迫以及 SARS-CoV-2 变异株的出现,疫苗对病毒感染的保护并不完善。在这里,我们采用 UDCA 作为 FXR 拮抗剂来调节 ACE2 的表达,ACE2 是 SARS-CoV-2 Delta 变种感染激活的关键途径之一。UDCA 是一种著名的肝脏保健品试剂,也是唯一获得临床批准的胆汁酸。在本文中,我们研究了 UDCA 对 Omicron 变异的保护功效,因为 UDCA 对 Delta 变异的保护作用此前已得到验证。当与感染了奥米克龙变异体的仓鼠组共同饲养导致自发的空气传播时,在感染后 4 天,预先服用 UDCA 的组相对于未服用 UDCA 的组体重减轻了 5%-10%。此外,UDCA 处理组鼻腔中 ACE2 的表达量减少了 3 倍,呼吸道中的病毒表达基因也减少了。这些数据表明,UDCA 可作为预防药物的另一种选择,它不仅能保护 SARS-CoV-2 不被 Delta 型变异体感染,还能保护其不受 Omicron 型变异体感染。我们的研究结果将有助于提出将 UDCA 从肝脏保健品重新定位为 SARS-CoV-2 感染的预防药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacology Research & Perspectives
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
5.30
自引率
3.80%
发文量
120
审稿时长
20 weeks
期刊介绍:
PR&P is jointly published by the American Society for Pharmacology and Experimental Therapeutics (ASPET), the British Pharmacological Society (BPS), and Wiley. PR&P is a bi-monthly open access journal that publishes a range of article types, including: target validation (preclinical papers that show a hypothesis is incorrect or papers on drugs that have failed in early clinical development); drug discovery reviews (strategy, hypotheses, and data resulting in a successful therapeutic drug); frontiers in translational medicine (drug and target validation for an unmet therapeutic need); pharmacological hypotheses (reviews that are oriented to inform a novel hypothesis); and replication studies (work that refutes key findings [failed replication] and work that validates key findings). PR&P publishes papers submitted directly to the journal and those referred from the journals of ASPET and the BPS
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


