Inhibition of Autophagy Alters Intracellular Transport of APP Resulting in Increased APP Processing
IF 2.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Alzheimer's disease (AD) pathology is characterized by amyloid beta (Aβ) plaques and dysfunctional autophagy. Aβ is generated by sequential proteolytic cleavage of amyloid precursor protein (APP), and the site of intracellular APP processing is highly debated, which may include autophagosomes. Here, we investigated the involvement of autophagy, including the role of ATG9 in APP intracellular trafficking and processing by applying the RUSH system, which allows studying the transport of fluorescently labeled mCherry‐APP‐EGFP in a systematic way, starting from the endoplasmic reticulum. HeLa cells, expressing the RUSH mCherry‐APP‐EGFP system, were investigated by live cell imaging, immunofluorescence, and Western blot. We found that mCherry‐APP‐EGFP passed through the Golgi faster in ATG9 knockout cells. Furthermore, ATG9 deletion shifted mCherry‐APP‐EGFP from early endosomes and lysosomes toward the plasma membrane concomitant with reduced endocytosis. Importantly, this alteration in mCherry‐APP‐EGFP transport resulted in increased secreted mCherry‐soluble APP and C‐terminal fragment‐EGFP. These effects were also phenocopied by pharmacological inhibition of ULK1, indicating that autophagy is regulating the intracellular trafficking and processing of APP. These findings contribute to the understanding of the role of autophagy in APP metabolism and could potentially have implications for new therapeutic approaches for AD.
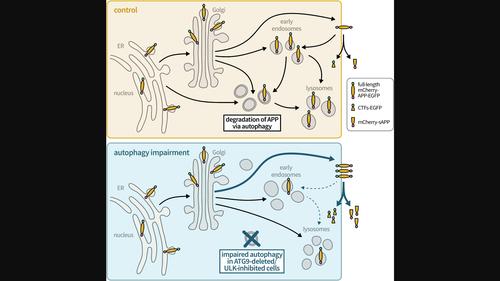
抑制自噬会改变 APP 的细胞内转运,导致 APP 处理过程增加
阿尔茨海默病(AD)的病理特征是淀粉样 beta(Aβ)斑块和自噬功能障碍。淀粉样β是由淀粉样前体蛋白(APP)的连续蛋白水解产生的,而细胞内APP的加工部位还存在很大争议,其中可能包括自噬体。RUSH系统可以从内质网开始系统地研究荧光标记的mCherry-APP-EGFP的转运,我们在此研究了自噬的参与,包括ATG9在APP胞内转运和处理中的作用。我们通过活细胞成像、免疫荧光和 Western 印迹对表达 RUSH mCherry-APP-EGFP 系统的 HeLa 细胞进行了研究。我们发现,在 ATG9 基因敲除的细胞中,mCherry-APP-EGFP 通过高尔基体的速度更快。此外,ATG9 基因缺失会使 mCherry-APP-EGFP 从早期内体和溶酶体转移到质膜,同时内吞作用也会减弱。重要的是,mCherry-APP-EGFP转运的这种改变导致分泌的mCherry-可溶性APP和C-末端片段-EGFP增加。药物抑制ULK1也会产生这些效应,这表明自噬正在调节APP的胞内转运和处理。这些发现有助于人们了解自噬在APP代谢中的作用,并有可能对AD的新治疗方法产生影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Traffic
生物-细胞生物学
CiteScore
8.10
自引率
2.20%
发文量
50
审稿时长
2 months
期刊介绍:
Traffic encourages and facilitates the publication of papers in any field relating to intracellular transport in health and disease. Traffic papers span disciplines such as developmental biology, neuroscience, innate and adaptive immunity, epithelial cell biology, intracellular pathogens and host-pathogen interactions, among others using any eukaryotic model system. Areas of particular interest include protein, nucleic acid and lipid traffic, molecular motors, intracellular pathogens, intracellular proteolysis, nuclear import and export, cytokinesis and the cell cycle, the interface between signaling and trafficking or localization, protein translocation, the cell biology of adaptive an innate immunity, organelle biogenesis, metabolism, cell polarity and organization, and organelle movement.
All aspects of the structural, molecular biology, biochemistry, genetics, morphology, intracellular signaling and relationship to hereditary or infectious diseases will be covered. Manuscripts must provide a clear conceptual or mechanistic advance. The editors will reject papers that require major changes, including addition of significant experimental data or other significant revision.
Traffic will consider manuscripts of any length, but encourages authors to limit their papers to 16 typeset pages or less.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


