Synthesis of α-Phenyl β-Enamino γ-Sultims: the New Horizon of the CSIC Reaction
IF 1.4
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we report the novel strategy for the synthesis of 4-enamino-5-phenyl-2,3-dihydroisothiazole 1-oxides (in other words α-phenyl β-enamino γ-sultims) based on the CSIC reaction. Particularly, readily available α-amino nitriles (the Strecker products) reacted with benzyl sulfinyl chloride to give the corresponding sulfinamides, which upon treatment with excess of LiHMDS converted into the target α-phenyl β-enamino γ-sultims. The method works well and tolerates strained 3- and 4-membered spirocyclic substituents. A preliminary in silico study indicated that the γ-sultim scaffold can be considered a promising pharmacophore template.
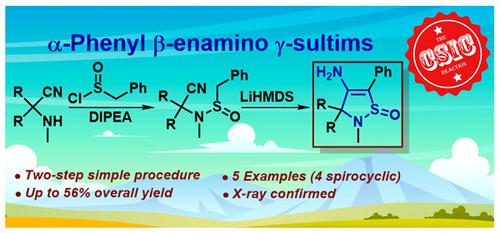
α-Phenyl β-Enamino γ-Sultims的合成:CSIC反应的新视野
在此,我们报告了基于 CSIC 反应合成 4-烯氨基-5-苯基-2,3-二氢异噻唑 1-氧化物(即 α-苯基 β-烯氨基 γ-亚砜)的新策略。特别是,现成的 α-氨基腈(Strecker 产物)与苄基亚磺酰氯反应生成相应的亚磺酰胺,这些亚磺酰胺经过量 LiHMDS 处理后转化为目标 α-苯基 β-烯氨基 γ-亚磺酸盐。该方法效果良好,并可容忍紧张的 3 元和 4 元螺环取代基。一项初步的硅学研究表明,γ-sultim 支架可被视为一种很有前景的药代模板。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


