Nuclear receptor corepressors non-canonically drive glucocorticoid receptor-dependent activation of hepatic gluconeogenesis
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
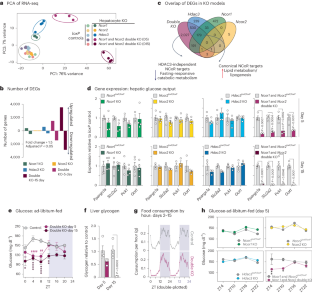
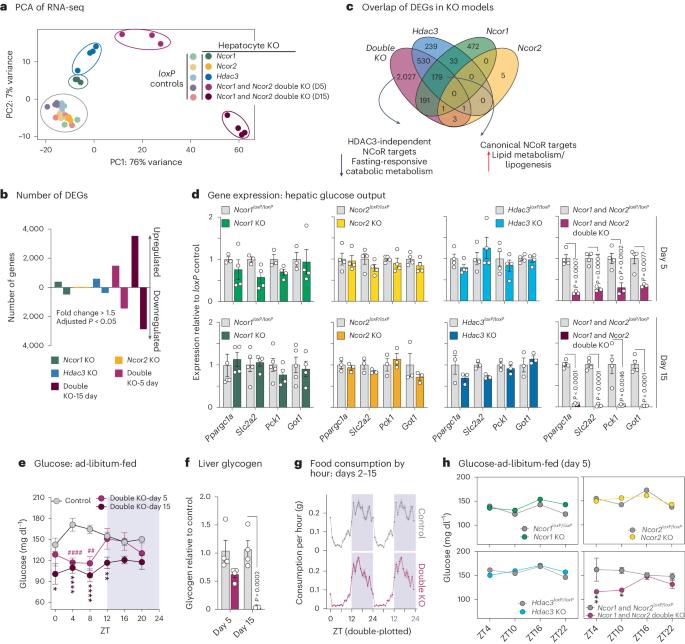
Nuclear receptor corepressors (NCoRs) function in multiprotein complexes containing histone deacetylase 3 (HDAC3) to alter transcriptional output primarily through repressive chromatin remodelling at target loci1–5. In the liver, loss of HDAC3 causes a marked hepatosteatosis largely because of de-repression of genes involved in lipid metabolism6,7; however, the individual roles and contribution of other complex members to hepatic and systemic metabolic regulation are unclear. Here we show that adult loss of both NCoR1 and NCoR2 (double knockout (KO)) in hepatocytes phenocopied the hepatomegalic fatty liver phenotype of HDAC3 KO. In addition, double KO livers exhibited a dramatic reduction in glycogen storage and gluconeogenic gene expression that was not observed with hepatic KO of individual NCoRs or HDAC3, resulting in profound fasting hypoglycaemia. This surprising HDAC3-independent activation function of NCoR1 and NCoR2 is due to an unexpected loss of chromatin accessibility on deletion of NCoRs that prevented glucocorticoid receptor binding and stimulatory effect on gluconeogenic genes. These studies reveal an unanticipated, non-canonical activation function of NCoRs that is required for metabolic health. Hauck et al. show that during fasting, nuclear receptor corepressors 1 and 2 act together to activate the transcription of target genes, which is critical for the physiological response to fasting in mice.
核受体核心抑制因子非调控性驱动糖皮质激素受体依赖性激活肝糖生成
核受体核心抑制因子(NCoRs)在含有组蛋白去乙酰化酶 3(HDAC3)的多蛋白复合物中发挥作用,主要通过靶基因座的抑制性染色质重塑来改变转录输出1,2,3,4,5。在肝脏中,HDAC3 的缺失会导致明显的肝脂肪变性,这主要是因为参与脂质代谢的基因受到了抑制6,7;然而,其他复合体成员在肝脏和全身代谢调控中的作用和贡献尚不清楚。在这里,我们发现,成年后肝细胞中 NCoR1 和 NCoR2 的缺失(双基因敲除(KO))表型与 HDAC3 KO 的肝巨细胞性脂肪肝表型相同。此外,双KO肝脏表现出糖原贮存和糖原生成基因表达的急剧减少,而肝脏单个NCoR或HDAC3的KO却没有观察到这种情况,这导致了严重的空腹低血糖症。NCoR1 和 NCoR2 这种令人惊讶的不依赖 HDAC3 的激活功能是由于 NCoRs 缺失时染色质可及性的意外丧失,从而阻止了糖皮质激素受体的结合和对糖原基因的刺激作用。这些研究揭示了新陈代谢健康所需的 NCoRs 意外的非经典激活功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


