Constructing and validating a risk model based on neutrophil-related genes for evaluating prognosis and guiding immunotherapy in colon cancer
Abstract
Background
Colon cancer is one of the most common digestive tract malignancies. Although immunotherapy has brought new hope to colon cancer patients, there is still a large proportion of patients who do not benefit from immunotherapy. Studies have shown that neutrophils can interact with immune cells and immune factors to affect the prognosis of patients.
Methods
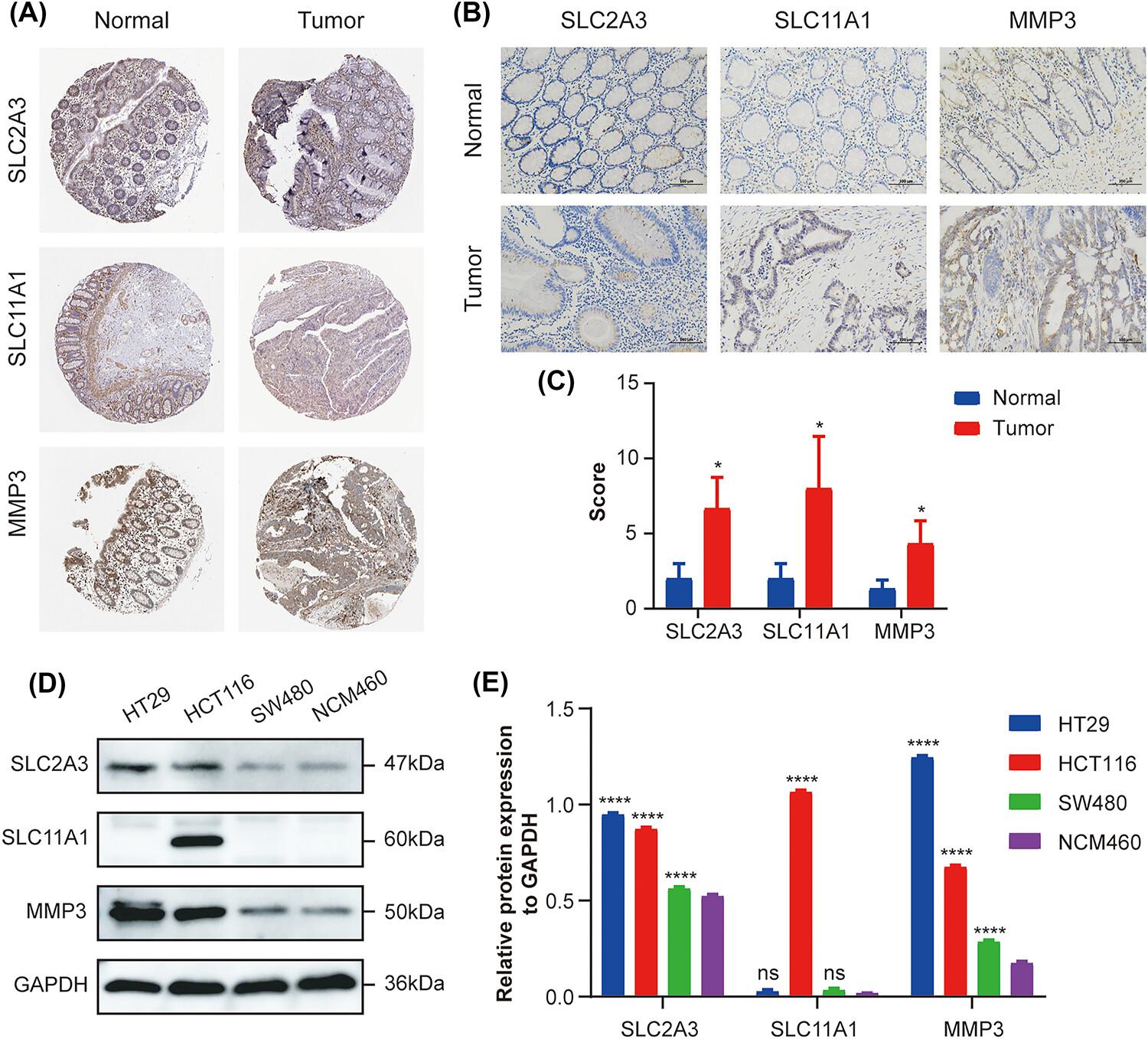
We first determined the infiltration level of neutrophils in tumors using the CIBERSORT algorithm and identified key genes in the final risk model by Spearman correlation analysis and subsequent Cox analysis. The risk score of each patient was obtained by multiplying the Cox regression coefficient and the gene expression level, and patients were divided into two groups based on the median of risk score. Differences in overall survival (OS) and progression-free survival (PFS) were assessed by Kaplan–Meier survival analysis, and model accuracy was validated in independent dataset. Differences in immune infiltration and immunotherapy were evaluated by immunoassay. Finally, immunohistochemistry and western blotting were performed to verify the expression of the three genes in the colon normal and tumor tissues.
Results
We established and validated a risk scoring model based on neutrophil-related genes in two independent datasets, The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) database, with SLC11A1 and SLC2A3 as risk factors and MMP3 as a protective factor. A new nomogram was constructed and validated by combining clinical characteristics and the risk score model to better predict patients OS and PFS. Immune analysis showed that patients in the high-risk group had immune cell infiltration level, immune checkpoint level and tumor mutational burden, and were more likely to benefit from immunotherapy.
Conclusions
The low-risk group showed better OS and PFS than the high-risk group in the neutrophil-related gene-based risk model. Patients in the high-risk group presented higher immune infiltration levels and tumor mutational burden and thus may be more responsive to immunotherapy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


