Chitosanase-immobilized magnetite-agar gel particles as a highly stable and reusable biocatalyst for enhanced production of physiologically active chitosan oligosaccharides
Abstract
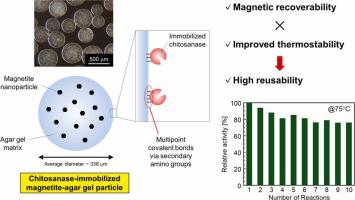
A novel immobilized chitosanase was developed and utilized to produce chitosan oligosaccharides (COSs) via chitosan hydrolysis. Magnetite-agar gel particles (average particle diameter: 338 μm) were prepared by emulsifying an aqueous agar solution dispersing 200-nm magnetite particles with isooctane containing an emulsifier at 80 °C, followed by cooling the emulsified mixture. The chitosanase from Bacillus pumilus was immobilized on the magnetite-agar gel particles chemically activated by introducing glyoxyl groups with high immobilization yields (>80%), and the observed specific activity of the immobilized chitosanase was 16% of that of the free enzyme. This immobilized chitosanase could be rapidly recovered from aqueous solutions by applying magnetic force. The thermal stability of the immobilized chitosanase improved remarkably compared with that of free chitosanase: the deactivation rate constants at 35 °C of the free and immobilized enzymes were 8.1 × 10−5 and 3.9 × 10−8 s−1, respectively. This immobilized chitosanase could be reused for chitosan hydrolysis at 75 °C and pH 5.6, and 80% of its initial activity was maintained even after 10 cycles of use. COSs with a degree of polymerization (DP) of 2–7 were obtained using this immobilized chitosanase, and the product content of physiologically active COSs (DP ≥ 5) reached approximately 50%.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


