Whole breast ultrafractionation radiotherapy after breast-conserving surgery in early breast cancer: A single-center, prospective, observational study from China
Abstract
Objective
This single-center, prospective, observational study was designed to investigate the toxicities, patient-reported outcome (PRO), and dosimetric analysis of whole breast ultrafractionation radiotherapy (RT) after breast-conserving surgery (BCS) in early breast cancer (BC).
Patients and methods
Patients diagnosed with BC stage I, II and treated with BCS were enrolled. A dose of 26 Gray (Gy) in five fractions was prescribed to the whole breast and tumor bed. Clinical endpoints included toxicities, PRO, and dosimetric analysis. PRO was measured by the European Organization for Research and Treatment of Cancer general quality of life questionnaire (EORTC QLQ-C30) and the BC-specific questionnaire (EORTC QLQ-BR23) questionnaires.
Results
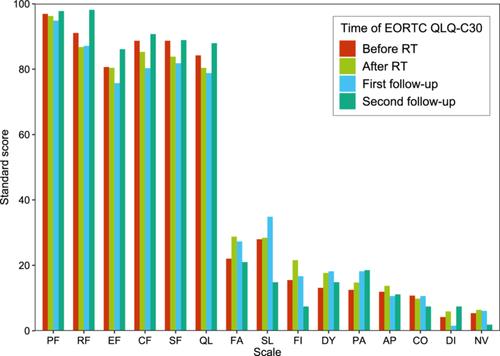
Between January 2022 and June 2023, 62 female patients were enrolled. The median age was 45 years. Most patients (83.9%) were diagnosed with pathological stage I disease. The median planning target volume (PTV) was 456.4 mL. The minimum, maximum, and mean doses, and D95 (dose of PTV irradiated volume more than 95%) to PTV were 20.2, 28.8, 27.2, and 26.3 Gy, respectively. The median mean lung dose and percentage lung volume receiving 8 Gy (V8) were 3.6 Gy and 13.4%, respectively. The median mean heart dose, V1.5 (percentage of organ volume irradiated with 1.5 Gy or higher), and V7 (percentage of organ volume irradiated with 7 Gy or higher) were 0.6 Gy, 6.8%, and 0.4%, respectively. Cosmetic effects before RT showed no obvious differences compared to that post RT. No toxicities of grade 3 or higher occurred. Five patients had asymptomatic radiation pneumonia (grade 1), and 12 patients had radiation dermatitis (grade 1). No factor was significantly related to radiation dermatitis or radiation pneumonia. For the EORTC QLQ-C30 and QLQ-BR23 questionnaires, all function and symptom scores before RT had no significant differences compared with that after RT, 1−2 months after RT, and 3−4 months after RT. Ultrafractionation RT did not worsen PRO. The 1-year crude local control was 100%.
Conclusion
Whole breast ultrafractionation RT after BCS in early BC has no severe toxicities and does not affect PRO. These results need to be further validated with a longer follow-up and a larger sample size.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


