EEG band power and phase-amplitude coupling in patients with Dravet syndrome
Abstract
Objective
Dravet syndrome (DS) is an epileptic encephalopathy caused by haploinsufficiency of the SCN1A gene. SCN1A gene deficiency limits the firing rates of fast-spiking inhibitory interneurons, which should reflect in abnormal aggregate network oscillatory electroencephalography (EEG) activity that can be measured by spectral power and phase-amplitude coupling (PAC) analysis. In this retrospective pilot study, we tested whether spectral EEG frequency band power and PAC metrics distinguish children with DS from age-matched controls, an early step toward establishing EEG markers of target engagement by gene or drug therapy.
Methods
EEG data were collected from patients with DS (N = 6) and age-matched control pediatric participants (N = 11) and analyzed for cumulative spectral power and PAC and classification capacity of these metrics, by logistic regression analysis. For this initial spectral and PAC analysis, we focused on sleep EEG, where myogenic artifact is minimal and where δ–γ and θ–γ coupling is otherwise expected to be robust.
Results
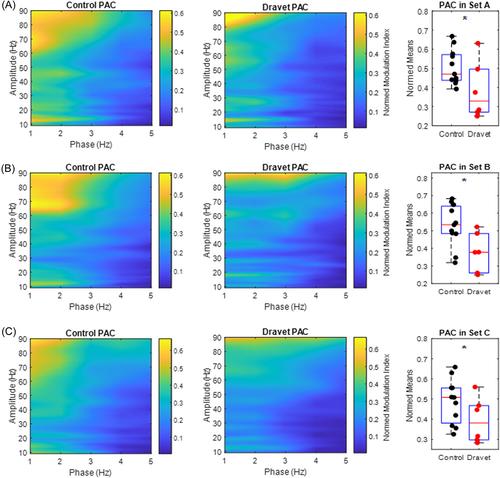
Cumulative δ (1– <4 Hz) and θ (4–7 Hz) power was significantly reduced in the DS group, compared with age-matched controls (p = 0.001 and p = 0.02, respectively). The δ power was a stronger classifier of separating DS from controls than θ power, with 87% and 83% accuracy, respectively. The γ power trended toward significant reduction (p = 0.08) in the DS group. We found significantly lower PAC between 1–2 Hz phase and 63–80 Hz amplitude in patients with DS compared with the age-matched controls (p = 0.003), with 78% classification accuracy between groups for PAC.
Interpretation
In this pilot study assessing EEG patterns during sleep, we found lower δ–θ power and PAC in patients with DS versus controls, which may reflect abnormal aggregate macroscale network communication patterns resulting from SCN1A deficiency. These measures may be useful metrics of therapeutic target engagement, particularly if the therapy restores the underlying DS pathophysiology. The sorting capacity of these metrics distinguished patients with DS from patients without DS and may in turn facilitate near-future development of disease and therapy target engagement biomarkers in this syndrome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


