Gastrointestinal infections and gastrointestinal haemorrhage are underestimated but serious adverse events in chimeric antigen receptor T-cell recipients: A real-world study
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
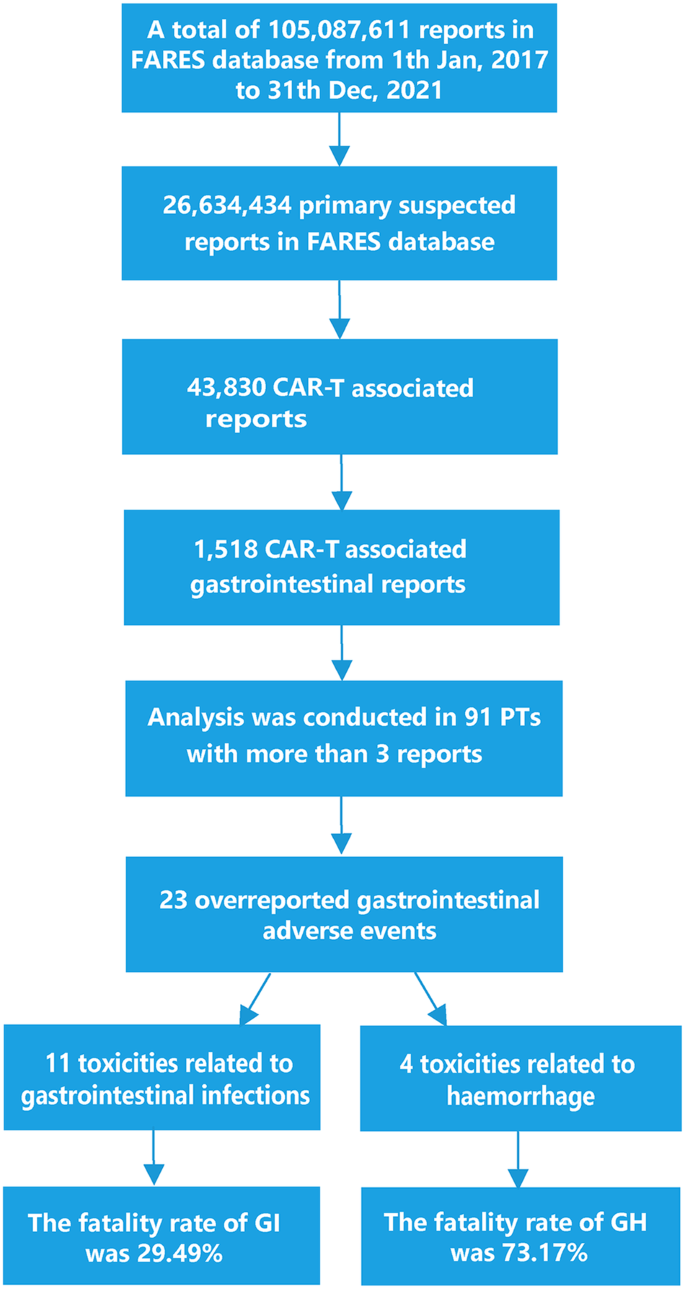
Chimeric antigen receptor T-cell (CAR-T) therapy has achieved durable response in patients with hematological malignancies, however, therapy-associated multisystem toxicities are commonly observed. Here, we systematically analyzed CAR-T-related gastrointestinal adverse events (GAEs) using the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) between January 2017 and December 2021. Disproportionality analyses were performed using reporting odds ratios (ROR) and information component (IC). Among 105,087,611 reports in FAERS, 1518 CAR-T-related GAEs reports were identified. 23 GAEs (n = 281, 18.51%) were significantly overreported following CAR-T therapy compared with the full database, of which 11 GAEs (n = 156, 10.28%) were associated with gastrointestinal infections (GI), such as clostridium difficile colitis (n = 44 [2.90%], ROR = 5.55), enterovirus infection (n = 23 [1.52%], ROR = 20.02), and mucormycosis (n = 15 [0.99%], ROR = 3.09). Overall, the fatality rate of 11 GI-related AEs was 29.49%, especially mucormycosis causing substantial mortality with 60%. In addition, 4 of 23 overreported GAEs were related to haemorrhage and the mortality of gastrointestinal haemorrhage was 73.17%. Lastly, 29 death-related GAEs were identified. These findings could help clinicians early alert those rarely reported but lethal GAEs, thus reducing the risk of severe toxicities.
在嵌合抗原受体 T 细胞受者中,胃肠道感染和胃肠道出血是被低估的严重不良事件:真实世界研究
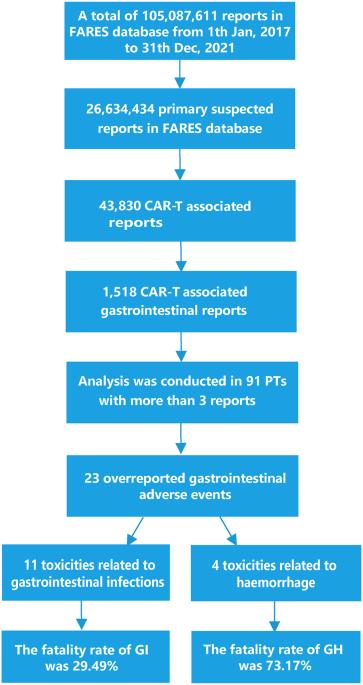
嵌合抗原受体 T 细胞(CAR-T)疗法已在血液恶性肿瘤患者中取得了持久的疗效,然而,与治疗相关的多系统毒性反应却屡见不鲜。在此,我们利用美国食品和药物管理局不良事件报告系统(FAERS)系统分析了2017年1月至2021年12月期间与CAR-T相关的胃肠道不良事件(GAEs)。使用报告几率比(ROR)和信息成分(IC)进行了比例失调分析。在 FAERS 的 105,087,611 份报告中,确定了 1518 份与 CAR-T 相关的 GAEs 报告。与完整数据库相比,CAR-T治疗后23例GAE(n = 281,18.51%)明显高报,其中11例GAE(n = 156,10.28%)与胃肠道感染(GI)有关,如艰难梭菌结肠炎(n = 44 [2.90%],ROR = 5.55)、肠道病毒感染(n = 23 [1.52%],ROR = 20.02)和粘液瘤病(n = 15 [0.99%],ROR = 3.09)。总体而言,11 种消化道相关急性症状的致死率为 29.49%,尤其是粘液瘤病导致的死亡率高达 60%。此外,23 例高报的 GAE 中有 4 例与出血有关,胃肠道出血的死亡率为 73.17%。最后,还发现了 29 例与死亡有关的 GAE。这些发现可以帮助临床医生及早警惕那些很少报告但却致命的 GAE,从而降低发生严重毒性反应的风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


