Adeno-associated virus genome quantification with amplification-free CRISPR-Cas12a
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Efficient manufacturing of recombinant Adeno-Associated Viral (rAAV) vectors to meet rising clinical demand remains a major hurdle. One of the most significant challenges is the generation of large amounts of empty capsids without the therapeutic genome. There is no standardized analytical method to accurately quantify the viral genes, and subsequently the empty-to-full ratio, making the manufacturing challenges even more complex. We propose the use of CRISPR diagnostics (CRISPR-Dx) as a robust and rapid approach to determine AAV genome titers. We designed and developed the CRISPR-AAV Evaluation (CRAAVE) assay to maximize sensitivity, minimize time-to-result, and provide a potentially universal design for quantifying multiple transgene constructs encapsidated within different AAV serotypes. We also demonstrate an on-chip CRAAVE assay with lyophilized reagents to minimize end user assay input. The CRAAVE assay was able to detect AAV titers as low as 7e7 vg/mL with high precision (<3% error) in quantifying unknown AAV titers when compared with conventional quantitative PCR (qPCR) method. The assay only requires 30 min of assay time, shortening the analytical workflow drastically. Our results suggest CRISPR-Dx could be a promising tool for efficient rAAV genome titer quantification and has the potential to revolutionize biomanufacturing process analytical technology (PAT).
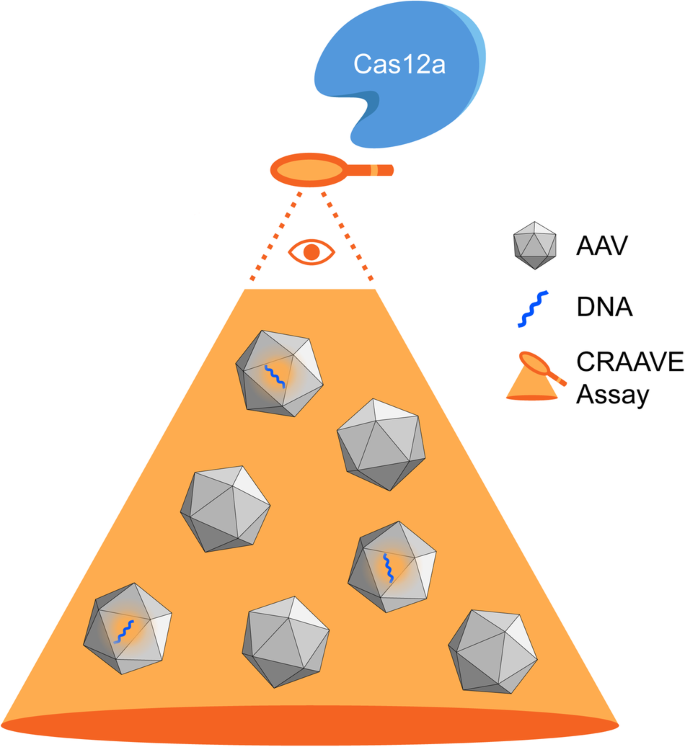
利用无扩增 CRISPR-Cas12a 进行腺相关病毒基因组定量。
高效生产重组腺相关病毒(rAAV)载体以满足日益增长的临床需求仍然是一个主要障碍。其中最重要的挑战之一是产生大量不含治疗基因组的空壳。目前还没有标准化的分析方法来准确量化病毒基因,以及随后的空-满比例,这使得生产挑战变得更加复杂。我们建议使用 CRISPR 诊断(CRISPR-Dx)作为一种稳健而快速的方法来确定 AAV 基因组滴度。我们设计并开发了 CRISPR-AAV 评估(CRAAVE)检测方法,以最大限度地提高灵敏度,缩短检测时间,并提供一种潜在的通用设计,用于量化不同 AAV 血清型中封装的多种转基因构建体。我们还展示了一种使用冻干试剂的片上 CRAAVE 检测方法,以尽量减少终端用户的检测投入。CRAAVE 检测法能够高精度检测低至 7e7 vg/mL 的 AAV 滴度 (
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


