Synergistic integration of histone deacetylase inhibitors apparently enhances the cytokine-induced killer cell efficiency in multiple myeloma via the NKG2D pathway
Abstract
Objectives
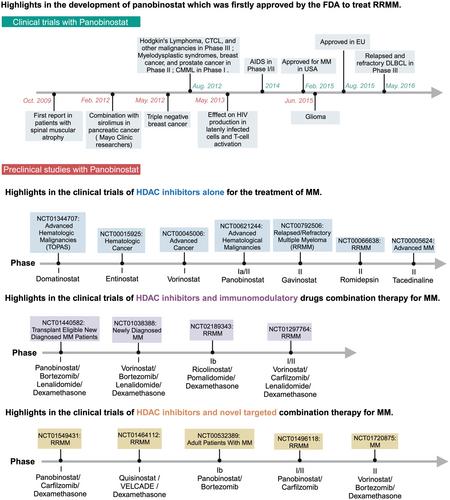
The rapid recognition of epigenetic manipulation's potential in restricting cancer cell capabilities spurred translational initiatives, including histone deacetylase inhibitors (HDACis). Clinical trials on multiple myeloma (MM) demonstrated substantial benefits of HDACis, coupled with promising outcomes from cytokine-induced killer cell (CIK) immunotherapy. Intriguingly, the unexplored synergy of HDACis and CIK cell immunotherapy in MM prompted our study.
Methods
We examined clinically relevant HDACis (panobinostat/LBH589 and romidepsin) alongside CIK cells derived from peripheral blood mononuclear cells across diverse MM cell lines (U266, RPMI8226, OPM-2 and NCI-H929). Utilising various in vitro methodologies, we investigated how HDACis enhance CIK cell lysis of myeloma cells through NKG2D/NKG2D ligand interactions.
Results
The results of our analysis indicated several key findings. (1) Enhanced cytotoxicity of CIK cells in MM cells when combined with HDACis. (2) Significant increase in apoptosis, suggesting HDACis and CIK may together enhance apoptotic effects in specific MM cell lines. (3) Elevated IFN-γ secretion and alterations in granzyme B secretion because of the independent activity of HDACis. (4) Notably, HDACis increased the expression of MICA/B and ULBP2, crucial for inducing antitumor cytotoxicity of NKT cells. Validation through NKG2D receptor blocking in CIK cells with a purified mouse antihuman NKG2D antibody further supported our findings.
Conclusions
Our analyses provide sufficient evidence to consider this clinically forgotten instance (HDACis-CIK cell combination) as a therapeutic priority for MM treatment. Furthermore, we suggest that NKG2D/NKG2D-ligand interactions activating NK/NKT cells may contribute to enhanced myeloma cell lysis in response to HDACis treatment by CIK cells.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


