The Electrochemical trans-Chloroformyloxylation of Unactivated Alkenes
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An attempted aryl selenium-catalyzed formation of cis-chlorohydrins from alkenes was unsuccessful but led to an electrochemical investigation for the trans-selective chloroformyloxylation of cyclic and acyclic alkenes in moderate to good yields. Interestingly, when 1,1-disubstituted alkenes were used, the corresponding vinyl chloride derivatives were obtained, and the application of 1-phenylcyclohex-1-ene led to the formation of an allyl chloride derivative.
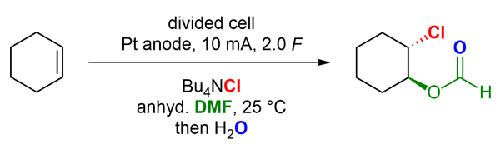
未活化烯烃的电化学反式氯甲酰氧基化反应
有人尝试用芳基硒催化烯烃形成顺式氯海德林,但没有成功,但却促成了一项电化学研究,即环烯和无环烯的反选择性氯甲酰氧基化反应,收率从中等到良好不等。有趣的是,当使用 1,1-二取代烯烃时,可得到相应的氯乙烯衍生物,而使用 1-苯基环己-1-烯可生成烯丙基氯衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


