Effect of alkali metal cation doping in graphitic carbon nitride towards photocatalytic generation of hydrogen peroxide under direct sunlight
IF 4.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
H2O2, a powerful oxidant with a vast number of industrial applications, is being synthesized by the energy intensive anthraquinone process. Recently, H2O2 has been synthesized using H2O and O2 by photocatalysis. Graphitic carbon nitride (g-C3N4) is a popular low-cost photocatalyst that is known to produce H2O2. However, the properties g-C3N4 needs to be tailored to improve the yield of H2O2. We discuss such a modification of g-C3N4 by alkali metal cation doping. Among the Li+, Na+ and K+ ions, doping of 10 wt% of K+ in g-C3N4 increase the yield of H2O2 (200 μmol g−1 h−1) by 2.5 times as compared un-doped g-C3N4 under natural sunlight.
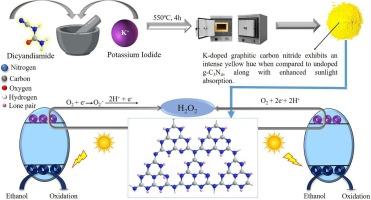
氮化石墨碳中掺杂碱金属阳离子对阳光直射下光催化生成过氧化氢的影响
HO 是一种强大的氧化剂,在工业上应用广泛,目前是通过高能耗的蒽醌工艺合成的。最近,人们通过光催化技术利用 HO 和 O 合成了 HO。氮化石墨碳(g-CN)是一种常用的低成本光催化剂,已知可以产生 HO。然而,需要对 g-CN 的特性进行调整,以提高 HO 的产量。我们讨论了通过掺杂碱金属阳离子对 g-CN 进行改性的问题。在 Li、Na 和 K 离子中,在 g-CN 中掺入 10 wt% 的 K,可将自然阳光下的 HO 产率(200 μmol g h)提高 2.5 倍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Communications
化学-物理化学
CiteScore
6.20
自引率
2.70%
发文量
183
审稿时长
46 days
期刊介绍:
Catalysis Communications aims to provide rapid publication of significant, novel, and timely research results homogeneous, heterogeneous, and enzymatic catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


