Ultralow interfacial tension achieved by extended anionic surfactants with a short hydrophobic chain
Abstract
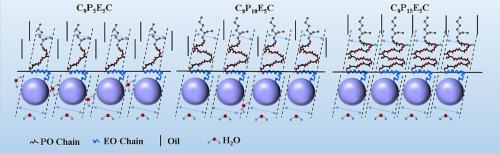
Extended surfactant is a new type of oil displacement agent with excellent performance in enhanced oil recovery (EOR). In this paper, the novel extended surfactants with short hydrophobe (ethylhexyl) have been studied. The dynamic interfacial tensions (IFTs) between extended surfactant (C8PxEyC) solutions and n-alkanes were measured by the spinning drop method. The effect of surfactant concentration, polyoxypropylene (PO) numbers, polyoxyethylene (EO) numbers and electrolyte on the IFTs were investigated. The experimental results show that the interfacial activity of the extended surfactants mainly depends on the PO groups, and the short hydrophobe extended anionic surfactant shows the similar behavior of reducing IFT to that of long alkyl chain one. The IFTs decrease with an increase of the PO numbers against n-alkanes, and an increase of the EO numbers leads to the destruction of the tight arrangement of the adsorption film because the size of EO group is smaller than that of PO group, which results in an increase of IFT. The addition of electrolyte can reduce the IFTs by weakening the electrostatic repulsion between surfactant molecules at the interface, but it has little effect on its HLB. The long EO chain of extended surfactant prevents divalent cation from binding two surfactant counterions, which results in the little effect on reducing IFT. This study is of great significance for the insight of extended surfactants and their application in high-salt reservoirs.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


