Biocatalytic enantioselective synthesis of cenobamate, an antiepileptic drug
IF 2.8
4区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
A green and efficient process for the synthesis of cenobamate has been accomplished in 70% yield and >99% ee through the bio-reduction of β-ketotetrazole using Daucus carota whole plant cells. The corresponding β-hydroxytetrazole was isolated in 60% yield and >98% ee. This is the first report on the biocatalytic reduction of β-ketotetrazole using plant enzymes derived from D. carota root cells with excellent enantioselectivity.
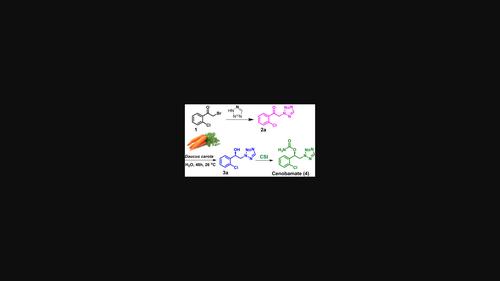
生物催化对映体选择性合成抗癫痫药物仙氨酰胺。
通过使用菊苣全株细胞对 β-酮四唑进行生物还原,完成了一种绿色、高效的苯甲酰氨基甲酸酯合成工艺,收率为 70%,ee >99%。相应的 β-羟基四氮唑的分离率为 60%,ee >98%。这是首次报道利用从胡萝卜根细胞中提取的植物酶生物催化还原β-酮四唑,并具有极佳的对映选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chirality
医学-分析化学
CiteScore
4.40
自引率
5.00%
发文量
124
审稿时长
1 months
期刊介绍:
The main aim of the journal is to publish original contributions of scientific work on the role of chirality in chemistry and biochemistry in respect to biological, chemical, materials, pharmacological, spectroscopic and physical properties.
Papers on the chemistry (physiochemical, preparative synthetic, and analytical), physics, pharmacology, clinical pharmacology, toxicology, and other biological aspects of chiral molecules will be published.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


