Synergy of de-walled Ganoderma Lucidum spore powder (GLSP) on targeted therapy in advanced non-squamous non-small cell lung cancer with epidermal growth factor receptor (EGFR) mutant: protocol for a randomized, double-blind, placebo-controlled study
IF 3.4
2区 医学
Q1 Medicine
引用次数: 0
Abstract
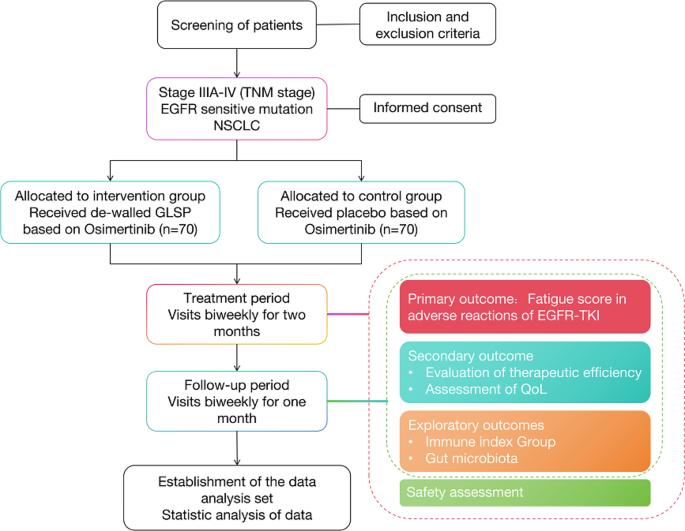
Osimertinib is regarded as a promising third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) for advanced non-squamous non-small cell lung cancer (NSCLC) patients who developed T790M. However the adverse effects, primarily fatigue, remain an overwhelming deficiency of Osimertinib, hindering it from achieving adequate clinical efficacy for such NSCLC. Ganoderma lucidum has been used for thousands of years in China to combat fatigue, while Ganoderma Lucidum spores powder (GLSP) is the main active ingredient. The aim of this study is to investigate whether GLSP is sufficiently effective and safe in improving fatigue and synergizing with Osimertinib in non-squamous NSCLC patients with EGFR mutant. A total of 140 participants will be randomly assigned to receive either de-walled GSLP or placebo for a duration of 56 days. The primary outcome measure is the fatigue score associated with EGFR-TKI adverse reactions at week 8, evaluated by the Chinese version of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire for Cancer Patients (QLQ-C30). Secondary outcomes include evaluation of treatment effectiveness, assessment of quality of life (QoL), and exploration of immune indicators and gut microbiota relationships. Following enrollment, visits are scheduled biweekly until week 12. China Clinical Trial Registry ChiCTR2300072786. Registrated on June 25, 2023.
去壁灵芝孢子粉(GLSP)对表皮生长因子受体(EGFR)突变的晚期非鳞状非小细胞肺癌靶向治疗的协同作用:随机、双盲、安慰剂对照研究方案
奥希替尼被认为是一种前景广阔的第三代表皮生长因子受体(EGFR)酪氨酸激酶抑制剂(TKI),适用于发生T790M的晚期非鳞状非小细胞肺癌(NSCLC)患者。然而,以疲劳为主的不良反应仍然是奥希替尼的一大缺陷,阻碍了它在此类 NSCLC 中取得足够的临床疗效。灵芝在中国用于消除疲劳已有数千年的历史,而灵芝孢子粉(GLSP)是其主要活性成分。本研究旨在探讨灵芝孢子粉在改善EGFR突变的非鳞癌NSCLC患者的疲劳状况以及与奥希替尼的协同作用方面是否足够有效和安全。共有 140 名参与者将被随机分配接受去壁 GSLP 或安慰剂治疗,疗程为 56 天。主要结局指标是第8周时与EGFR-TKI不良反应相关的疲劳评分,由欧洲癌症研究和治疗组织(EORTC)癌症患者生活质量问卷(QLQ-C30)的中文版进行评估。次要结果包括治疗效果评估、生活质量(QoL)评估以及免疫指标与肠道微生物群关系的探讨。入组后,每两周进行一次访视,直至第 12 周。中国临床试验注册中心 ChiCTR2300072786。注册日期:2023 年 6 月 25 日。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

BMC Complementary and Alternative Medicine
INTEGRATIVE & COMPLEMENTARY MEDICINE-
CiteScore
7.00
自引率
0.00%
发文量
0
审稿时长
3 months
期刊介绍:
BMC Complementary Medicine and Therapies is an open access journal publishing original peer-reviewed research articles on interventions and resources that complement or replace conventional therapies, with a specific emphasis on research that explores the biological mechanisms of action, as well as their efficacy, safety, costs, patterns of use and/or implementation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


