Genetic and geographic population structure in the malaria vector, Anopheles farauti, provides a candidate system for pioneering confinable gene-drive releases
IF 3.9
2区 生物学
Q2 ECOLOGY
引用次数: 0
Abstract
Indoor insecticide applications are the primary tool for reducing malaria transmission in the Solomon Archipelago, a region where Anopheles farauti is the only common malaria vector. Due to the evolution of behavioural resistance in some An. farauti populations, these applications have become less effective. New malaria control interventions are therefore needed in this region, and gene-drives provide a promising new technology. In considering developing a population-specific (local) gene-drive in An. farauti, we detail the species’ population genetic structure using microsatellites and whole mitogenomes, finding many spatially confined populations both within and between landmasses. This strong population structure suggests that An. farauti would be a useful system for developing a population-specific, confinable gene-drive for field release, where private alleles can be used as Cas9 targets. Previous work on Anopheles gambiae has used the Cardinal gene for the development of a global population replacement gene-drive. We therefore also analyse the Cardinal gene to assess whether it may be a suitable target to engineer a gene-drive for the modification of local An. farauti populations. Despite the extensive population structure observed in An. farauti for microsatellites, only one remote island population from Vanuatu contained fixed and private alleles at the Cardinal locus. Nonetheless, this study provides an initial framework for further population genomic investigations to discover high-frequency private allele targets in localized An. farauti populations. This would enable the development of gene-drive strains for modifying localised populations with minimal chance of escape and may provide a low-risk route to field trial evaluations.
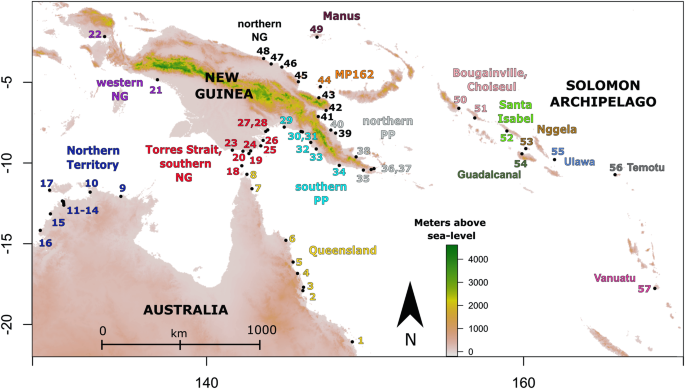
疟疾病媒 Anopheles farauti 的遗传和地理种群结构为开创可封闭基因驱动释放提供了一个候选系统。
在所罗门群岛,法罗帝疟蚊是唯一常见的疟疾病媒,而室内喷洒杀虫剂是减少疟疾传播的主要手段。由于一些法氏疟蚊种群产生了行为抗药性,室内杀虫剂的使用效果已大打折扣。因此,该地区需要新的疟疾控制干预措施,而基因驱动技术提供了一种前景广阔的新技术。在考虑开发法氏疟原虫种群特异性(本地)基因驱动时,我们使用微卫星和整个有丝分裂基因组详细研究了该物种的种群遗传结构,发现在陆地内部和陆地之间存在许多空间局限性种群。这种强大的种群结构表明,法氏疟蚊将是一个有用的系统,可用于开发种群特异性的、可限制的基因驱动,以进行野外释放,其中私有等位基因可用作 Cas9 靶标。此前有关冈比亚按蚊的研究利用卡迪纳尔基因开发了全球种群替代基因驱动。因此,我们也对卡迪纳尔基因进行了分析,以评估它是否适合作为改造当地法氏疟蚊种群的基因驱动的目标。尽管在法氏鳗的微卫星上观察到了广泛的种群结构,但只有一个来自瓦努阿图的偏远岛屿种群在Cardinal基因座上含有固定的等位基因。尽管如此,这项研究为进一步的种群基因组调查提供了一个初步框架,以发现法劳提蚁局部种群中的高频私人等位基因目标。这将使基因驱动品系的开发成为可能,从而以最小的逃逸几率改造局部种群,并为田间试验评估提供了一条低风险途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Heredity
生物-进化生物学
CiteScore
7.50
自引率
2.60%
发文量
84
审稿时长
4-8 weeks
期刊介绍:
Heredity is the official journal of the Genetics Society. It covers a broad range of topics within the field of genetics and therefore papers must address conceptual or applied issues of interest to the journal''s wide readership
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


