A review of real-world evidence on preemptive pharmacogenomic testing for preventing adverse drug reactions: a reality for future health care
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
Abstract
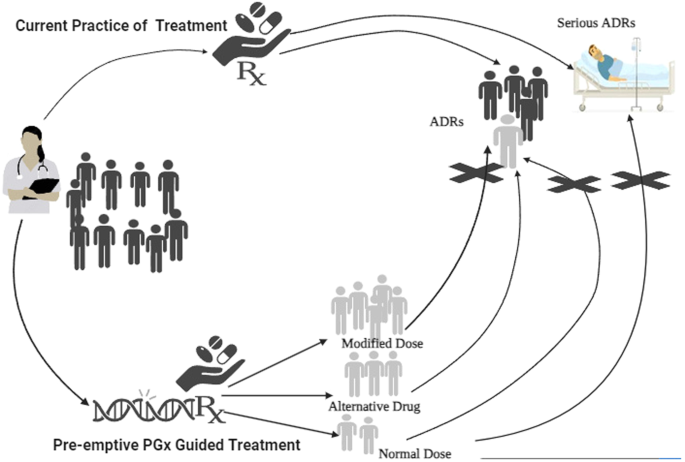
Adverse drug reactions (ADRs) are a significant public health concern and a leading cause of hospitalization; they are estimated to be the fourth leading cause of death and increasing healthcare costs worldwide. Carrying a genetic variant could alter the efficacy and increase the risk of ADRs associated with a drug in a target population for commonly prescribed drugs. The use of pre-emptive pharmacogenetic/omic (PGx) testing can improve drug therapeutic efficacy, safety, and compliance by guiding the selection of drugs and/or dosages. In the present narrative review, we examined the current evidence of pre-emptive PGx testing-based treatment for the prevention of ADRs incidence and hospitalization or emergency department visits due to serious ADRs, thus improving patient safety. We then shared our perspective on the importance of preemptive PGx testing in clinical practice for the safe use of medicines and decreasing healthcare costs.
关于预防药物不良反应的先期药物基因组学检测的现实证据综述:未来医疗保健的现实。
药物不良反应(ADRs)是一个重大的公共卫生问题,也是导致住院治疗的一个主要原因;据估计,它是导致死亡的第四大原因,并增加了全球的医疗成本。对于常用处方药的目标人群来说,携带基因变异可能会改变药效并增加与药物相关的不良反应风险。使用先期药物遗传学/基因组学(PGx)检测可通过指导药物和/或剂量的选择,提高药物的疗效、安全性和依从性。在本综述中,我们研究了目前基于先期药物基因组学检测的治疗方法在预防药物不良反应发生率和严重药物不良反应导致的住院或急诊就诊,从而提高患者安全性方面的证据。然后,我们就临床实践中抢先进行 PGx 试验对于安全用药和降低医疗成本的重要性分享了自己的观点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


