Advancing rare disease treatment: EMA’s decade-long insights into engineered adoptive cell therapy for rare cancers and orphan designation
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
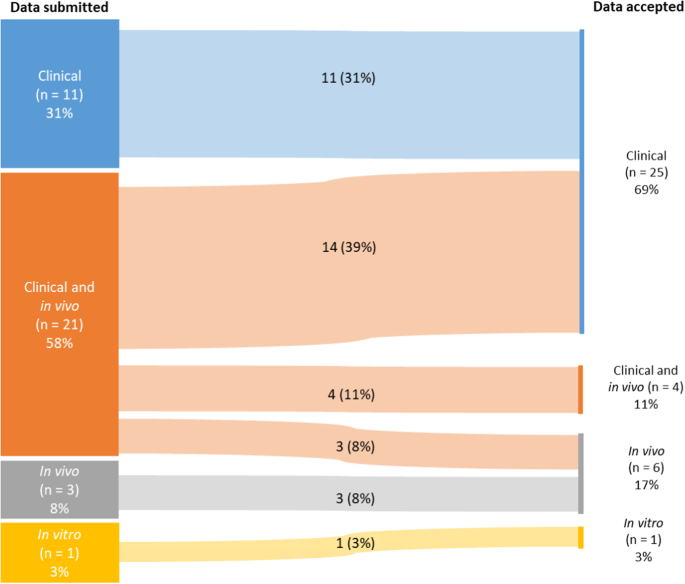
Adoptive cell therapy (ACT), particularly chimeric antigen receptor (CAR)-T cell therapy, has emerged as a promising approach for targeting and treating rare oncological conditions. The orphan medicinal product designation by the European Union (EU) plays a crucial role in promoting development of medicines for rare conditions according to the EU Orphan Regulation. This regulatory landscape analysis examines the evolution, regulatory challenges, and clinical outcomes of genetically engineered ACT, with a focus on CAR-T cell therapies, based on the European Medicines Agency’s Committee for Orphan Medicinal Products review of applications evaluated for orphan designation and maintenance of the status over a 10-year period. In total, 30 of 36 applications were granted an orphan status, and 14 subsequently applied for maintenance of the status at time of marketing authorisation or extension of indication. Most of the products were autologous cell therapies using a lentiviral vector and were developed for the treatment of rare haematological B-cell malignancies. The findings revealed that 80% (29/36) of the submissions for orphan designation were supported by preliminary clinical data showing a potential efficacy of the candidate products and an added clinical benefit over currently authorised medicines for the proposed orphan condition. Notably, in 89% (32/36) of the cases significant benefit of the new products was accepted based on a clinically relevant advantage over existing therapies. Twelve of fourteen submissions reviewed for maintenance of the status at time of marketing authorisation or extension of indication demonstrated significant benefit of the products over existing satisfactory methods of treatment within the approved therapeutic indications, but one of the applications was withdrawn during the regulatory evaluation. This article summarises the key findings related to the use of engineered ACT, primarily CAR-T cell therapies, in targeting and treating rare cancers in the EU. It emphasises the importance of use of clinical data in supporting medical plausibility and significant benefit at the stage of orphan designation and highlights the high success rate for these products in obtaining initial orphan designations and subsequent maintaining the status at the time of marketing authorisation or extension of indication.
推进罕见病治疗:EMA 十年来对罕见癌症的工程化收养细胞疗法和孤儿指定的见解。
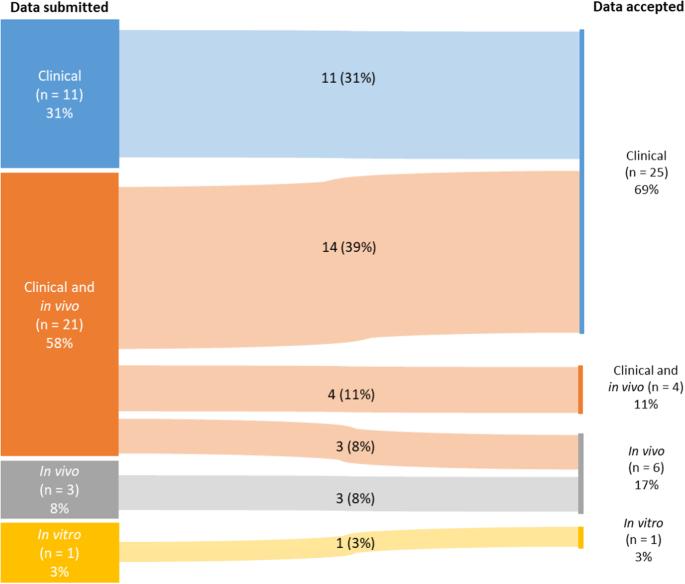
采用细胞疗法(ACT),尤其是嵌合抗原受体(CAR)-T 细胞疗法,已成为针对和治疗罕见肿瘤疾病的一种前景广阔的方法。根据《欧盟孤儿条例》(EU Orphan Regulation),欧盟(EU)指定的孤儿药在促进罕见病药物的开发方面发挥着至关重要的作用。本监管情况分析报告根据欧洲药品管理局孤儿药产品委员会在 10 年内对孤儿药指定和孤儿药地位维持申请的评估审查,研究了基因工程 ACT 的演变、监管挑战和临床结果,重点关注 CAR-T 细胞疗法。在 36 项申请中,共有 30 项被授予孤儿地位,14 项随后在获得上市许可或扩大适应症时申请维持孤儿地位。这些产品大多是使用慢病毒载体的自体细胞疗法,用于治疗罕见的血液 B 细胞恶性肿瘤。研究结果表明,80%(29/36)的孤儿药申请都有初步临床数据支持,这些数据显示候选产品具有潜在疗效,而且与目前已获授权的药物相比,对拟议的孤儿病症具有更多临床益处。值得注意的是,在 89%(32/36)的案例中,新产品的显著疗效是基于与现有疗法相比的临床相关优势而被认可的。本文总结了欧盟在使用工程 ACT(主要是 CAR-T 细胞疗法)靶向治疗罕见癌症方面的主要发现。文章强调了在孤儿指定阶段使用临床数据支持医学合理性和显著疗效的重要性,并着重介绍了这些产品在获得初始孤儿指定以及随后在上市授权或扩大适应症时保持该地位的高成功率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


