下载PDF
{"title":"Patterns of structural variants within TP53 introns and relocation of the TP53 promoter: a commentary†","authors":"Hannah C Beird, Dimitri Lin, Alexander J Lazar, P Andrew Futreal","doi":"10.1002/path.6270","DOIUrl":null,"url":null,"abstract":"<p>Gene disruption from double-strand DNA breaks within introns is a mechanism of inactivating the tumor suppressor <i>TP53</i>. This occurs more frequently in osteosarcoma and biliary adenocarcinoma compared with other cancer types. The patterns of intron breakpoints within <i>TP53</i> do not correlate with prevalence, intron length, or overall genome-wide levels of rearrangements. Therefore, these breakpoints appear to be selected for reasons other than to disrupt <i>TP53</i>. A recent article published by Saba <i>et al</i> in <i>The Journal of Pathology</i> illustrates a benefit to having breakpoints within intron 1 using high-quality matched genomic and transcriptomic osteosarcoma sequencing data as well as <i>in vitro</i> validation. The authors describe how the rearrangement results in relocation of the <i>TP53</i> promoter region to regions upstream of genes that encode members of cartilage, growth plate development, osteoclast formation, and other <i>TP53</i>-related pathways. The upregulation of these genes by the <i>TP53</i> promoter are gain-of-function events that are likely to promote tumor development and growth. Therefore, this article presents a potential new paradigm in which a single mutation would result in both the loss of a tumor suppressor and the gain of an oncogenic program. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 2","pages":"131-134"},"PeriodicalIF":5.6000,"publicationDate":"2024-03-14","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6270","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6270","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
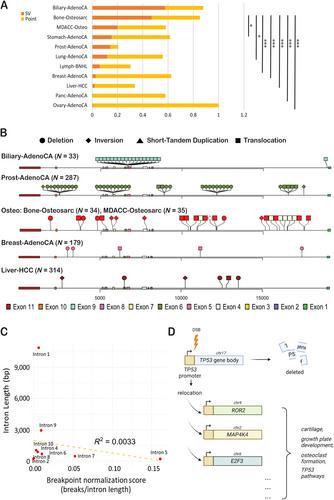
Gene disruption from double-strand DNA breaks within introns is a mechanism of inactivating the tumor suppressor TP53 . This occurs more frequently in osteosarcoma and biliary adenocarcinoma compared with other cancer types. The patterns of intron breakpoints within TP53 do not correlate with prevalence, intron length, or overall genome-wide levels of rearrangements. Therefore, these breakpoints appear to be selected for reasons other than to disrupt TP53 . A recent article published by Saba et al in The Journal of Pathology illustrates a benefit to having breakpoints within intron 1 using high-quality matched genomic and transcriptomic osteosarcoma sequencing data as well as in vitro validation. The authors describe how the rearrangement results in relocation of the TP53 promoter region to regions upstream of genes that encode members of cartilage, growth plate development, osteoclast formation, and other TP53 -related pathways. The upregulation of these genes by the TP53 promoter are gain-of-function events that are likely to promote tumor development and growth. Therefore, this article presents a potential new paradigm in which a single mutation would result in both the loss of a tumor suppressor and the gain of an oncogenic program. © 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
TP53 内含子结构变异的模式和 TP53 启动子的迁移:评论†。
内含子内双链 DNA 断裂造成的基因破坏是使肿瘤抑制因子 TP53 失活的一种机制。与其他癌症类型相比,这种情况在骨肉瘤和胆道腺癌中发生得更为频繁。TP53 内含子断点的模式与患病率、内含子长度或全基因组重排的总体水平无关。因此,选择这些断点的原因似乎与破坏 TP53 无关。Saba 等人最近在《病理学杂志》(The Journal of Pathology)上发表的一篇文章利用高质量的匹配基因组和转录组骨肉瘤测序数据以及体外验证,说明了在内含子 1 中设置断点的好处。作者描述了重排如何导致 TP53 启动子区域迁移到编码软骨、生长板发育、破骨细胞形成和其他 TP53 相关通路的基因上游区域。TP53 启动子对这些基因的上调属于功能增益事件,很可能会促进肿瘤的发育和生长。因此,本文提出了一种潜在的新范式,即单个突变会导致肿瘤抑制因子的缺失和致癌程序的获得。© 2024 作者。病理学杂志》由约翰威利父子有限公司代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


