Neutrophil ALDH2 is a new therapeutic target for the effective treatment of sepsis-induced ARDS
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
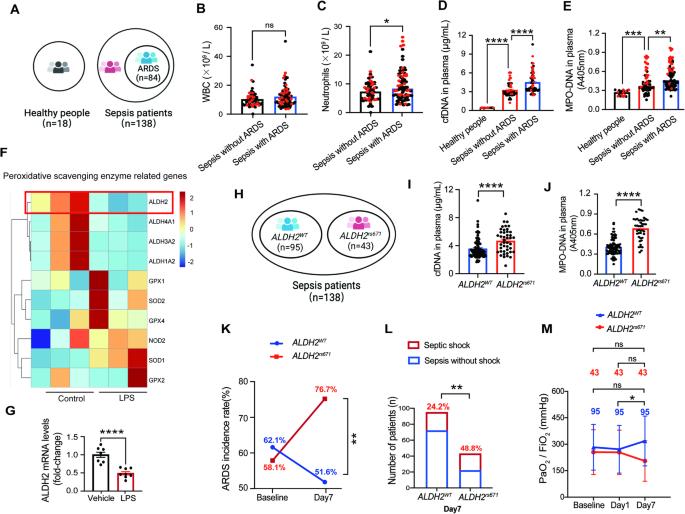
Acetaldehyde dehydrogenase 2 (ALDH2) mutations are commonly found in a subgroup of the Asian population. However, the role of ALDH2 in septic acute respiratory distress syndrome (ARDS) remains unknown. Here, we showed that human subjects carrying the ALDH2rs671 mutation were highly susceptible to developing septic ARDS. Intriguingly, ALDH2rs671-ARDS patients showed higher levels of blood cell-free DNA (cfDNA) and myeloperoxidase (MPO)-DNA than ALDH2WT-ARDS patients. To investigate the mechanisms underlying ALDH2 deficiency in the development of septic ARDS, we utilized Aldh2 gene knockout mice and Aldh2rs671 gene knock-in mice. In clinically relevant mouse sepsis models, Aldh2-/- mice and Aldh2rs671 mice exhibited pulmonary and circulating NETosis, a specific process that releases neutrophil extracellular traps (NETs) from neutrophils. Furthermore, we discovered that NETosis strongly promoted endothelial destruction, accelerated vascular leakage, and exacerbated septic ARDS. At the molecular level, ALDH2 increased K48-linked polyubiquitination and degradation of peptidylarginine deiminase 4 (PAD4) to inhibit NETosis, which was achieved by promoting PAD4 binding to the E3 ubiquitin ligase CHIP. Pharmacological administration of the ALDH2-specific activator Alda-1 substantially alleviated septic ARDS by inhibiting NETosis. Together, our data reveal a novel ALDH2-based protective mechanism against septic ARDS, and the activation of ALDH2 may be an effective treatment strategy for sepsis.
中性粒细胞 ALDH2 是有效治疗败血症诱发的 ARDS 的新治疗靶点。
乙醛脱氢酶 2(ALDH2)突变常见于亚裔人群。然而,ALDH2 在脓毒症急性呼吸窘迫综合征(ARDS)中的作用仍然未知。在这里,我们发现携带 ALDH2rs671 突变的受试者极易患脓毒性急性呼吸窘迫综合征。耐人寻味的是,与 ALDH2WT-ARDS 患者相比,ALDH2rs671-ARDS 患者的血中无细胞 DNA(cfDNA)和髓过氧化物酶(MPO)-DNA 水平更高。为了研究脓毒症 ARDS 发生的 ALDH2 缺乏机制,我们利用了 Aldh2 基因敲除小鼠和 Aldh2rs671 基因敲入小鼠。在临床相关的小鼠脓毒症模型中,Aldh2-/-小鼠和 Aldh2rs671 小鼠表现出肺部和循环中的中性粒细胞胞外捕获物(NET)增多症,这是一种从中性粒细胞释放中性粒细胞胞外捕获物(NET)的特殊过程。此外,我们还发现,NETosis 能强烈促进内皮破坏、加速血管渗漏并加重脓毒性 ARDS。在分子水平上,ALDH2 增加了 K48 链接的多泛素化和肽精氨酸脱氨酶 4(PAD4)的降解,从而抑制了 NETosis,这是通过促进 PAD4 与 E3 泛素连接酶 CHIP 的结合实现的。通过抑制NETosis,ALDH2特异性激活剂Alda-1的药理作用大大缓解了脓毒性ARDS。总之,我们的数据揭示了一种基于 ALDH2 的新型脓毒性 ARDS 保护机制,激活 ALDH2 可能是一种有效的脓毒症治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


