Engagement of sialylated glycans with Siglec receptors on suppressive myeloid cells inhibits anticancer immunity via CCL2
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
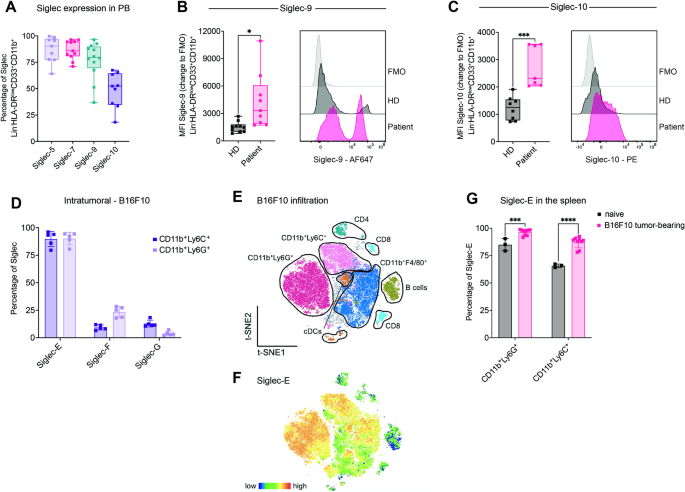
The overexpression of sialic acids on glycans, called hypersialylation, is a common alteration found in cancer cells. Sialylated glycans can enhance immune evasion by interacting with sialic acid-binding immunoglobulin-like lectin (Siglec) receptors on tumor-infiltrating immune cells. Here, we investigated the effect of sialylated glycans and their interaction with Siglec receptors on myeloid-derived suppressor cells (MDSCs). We found that MDSCs derived from the blood of lung cancer patients and tumor-bearing mice strongly express inhibitory Siglec receptors and are highly sialylated. In murine cancer models of emergency myelopoiesis, Siglec-E knockout in myeloid cells resulted in prolonged survival and increased tumor infiltration of activated T cells. Targeting suppressive myeloid cells by blocking Siglec receptors or desialylation strongly reduced their suppressive potential. We further identified CCL2 as a mediator involved in T-cell suppression upon interaction between sialoglycans and Siglec receptors on MDSCs. Our results demonstrated that sialylated glycans inhibit anticancer immunity by modulating CCL2 expression.

抑制性髓系细胞上的 Siglec 受体与ialylated 聚糖结合,可通过 CCL2 抑制抗癌免疫。
聚糖上的硅酸过度表达(称为超硅酸化)是癌细胞中常见的一种改变。硅烷基化聚糖可通过与肿瘤浸润免疫细胞上的硅烷基酸结合免疫球蛋白样凝集素(Siglec)受体相互作用来增强免疫逃避能力。在这里,我们研究了硅烷基化聚糖及其与 Siglec 受体相互作用对髓源性抑制细胞(MDSCs)的影响。我们发现,从肺癌患者和肿瘤小鼠血液中提取的 MDSCs 强烈表达抑制性 Siglec 受体,并且高度糖基化。在紧急骨髓生成的小鼠癌症模型中,敲除骨髓细胞中的 Siglec-E 可延长存活时间,并增加活化 T 细胞的肿瘤浸润。通过阻断 Siglec 受体或去淀粉酰化来靶向抑制性髓系细胞,可大大降低其抑制潜能。我们进一步确定了 CCL2 是一种参与 T 细胞抑制的介质,它通过 Sialoglyc 与 MDSCs 上的 Siglec 受体相互作用而发挥作用。我们的研究结果表明,糖基化聚糖通过调节 CCL2 的表达抑制了抗癌免疫。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


