Development of a functional outcome measure for riboflavin transporter deficiency
Abstract
Background and Aims
Riboflavin transporter deficiency (RTD) is a progressive inherited neuropathy of childhood onset, characterised clinically by pontobulbar palsy, sensory ataxia, sensorineural deafness, muscle weakness, optic atrophy and respiratory failure. A robust and responsive functional outcome measure is essential for future clinical trials of disease-modifying therapies including genetic therapies. The Charcot–Marie–Tooth disease Pediatric Scale (CMTPedS) is a well-validated outcome measure for CMT and related neuropathies, and might have utility for measuring disease progression in individuals with RTD. However, the CMTPedS requires modifications to account for phenotypic differences between children with CMT and RTD. The aim of this study was to develop a functional outcome measure based on the CMTPedS for specific use in individuals with RTD.
Methods
The CMTPedS data collected over the last 10 years in individuals with RTD attending the Peripheral Neuropathy Management Clinic at the Children's Hospital at Westmead (Sydney, Australia) were reviewed to evaluate each item within the CMTPedS. A literature review of articles published until September 2021 for functional outcome measures generated an item pool for pilot testing. The results of this pilot testing, alongside analysis of existing CMTPedS item scores in the RTD cohort, informed the modification of the CMTPedS.
Results
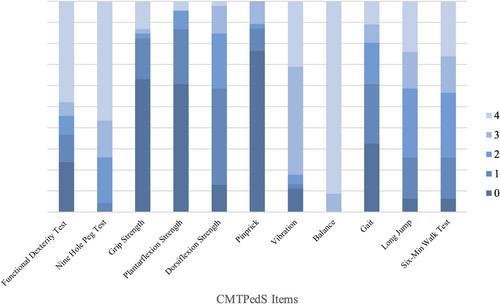
CMTPedS data were reviewed for eight individuals over the past 10 years. Two items were identified as requiring modification or removal and additional items of proximal strength and function needed to be considered. Six studies were identified in the literature review, and five items were selected for pilot testing. ‘Shoulder internal rotation’ and the ‘30-s sit to stand test’ were added as proximal measures of strength and function. The composite balance item comprising nine tasks in the CMTPedS showed a ceiling effect and was replaced with the single ‘Feet apart on a line eyes open’ balance item. ‘Pinprick sensation’ was removed due to a floor effect.
Interpretation
This study provides preliminary evidence that the Riboflavin Transporter Deficiency Pediatric Scale (RTDPedS) is a functional outcome measure covering strength, upper and lower limb function, balance and mobility for individuals with RTD to assess disease severity and progression in clinical trials and cohort studies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


