Experimental Investigation and Thermodynamic Assessment of the Cr–Ti System
Abstract
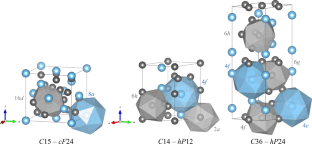
The Cr–Ti system was investigated by several experimental methods and first-principles calculations. The thermodynamic activity of the body-centered cubic solid solution was measured by Knudsen effusion mass spectrometry. The stability of all three polymorphic structures of the Laves phase (C14, C15, and C36) was determined by differential thermal analysis, and the equilibrium tie-lines with the solid solution were obtained by combining results from diffusion couples and equilibrated alloys. The enthalpy of formation of the Laves phases with the corresponding end-members were calculated using density functional theory and the obtained values were integrated in the models. The experimental and computed data available in the literature was reviewed and the binary system was assessed by the Calphad method. The present evaluation results in an improved thermodynamic description, which can describe the experimentally observed activity in a large temperature range. The temperatures of the invariant reactions between the C15 and the C36 phase with the Cr-rich and the Ti-rich bcc solid solution were significantly modified. The difference of the temperature of transformation between the C15 and the C36 polytypes on both sides of the Laves phase is much smaller than reported previously.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


