Forensic implications of novel synthesis of cathinone derivatives by Neber and modified Neber rearrangements
Abstract
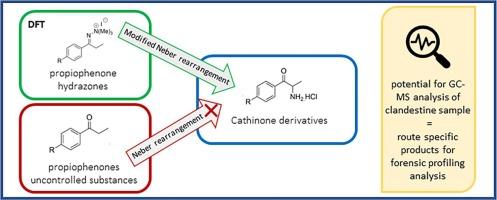
Cathinone and its synthetic analogues are known compounds of clandestine interest. Investigation into novel pathways for synthesising cathinone derivatives has potential for forensic analysis and tracking. The known Neber rearrangement of commercially available phenylpropanones that were evaluated yielded amides described herein and was not viable for clandestine synthesis of cathinone derivatives. Whereas the known modified Neber rearrangement of phenylpropanones that were evaluated via stable aziridine salts and subsequent treatment with acid, gave cathinone derivatives described herein in poor to low yields (2–17%). Assessment of the reagents, equipment, and procedures required for the modified Neber rearrangement was considered as only viable for more advanced clandestine operations. An improved understanding of the potential by-product formation from the modified Neber rearrangement was determined by density functional theory (DFT) of hydrazone to azirine to aziridine intermediates and attempted dynamic NMR spectroscopy of a hydrazone described herein. The substantially lower energy of the azirine step compared to the starting hydrazonium salt step of the reaction mechanism implied that the azirine structure was a short-lived intermediate, and unable to be experimentally determined. New mass-spectral fragmentation data of compounds described herein was reported, where differentiation was observed for some individual compounds at the GC-EIMS fragmentation pattern level. From this study, individual mass-spectrometry fragmentation of key compounds evaluated from the modified Neber rearrangement of commercially available phenylpropanones indicates potential for forensic profiling analysis applications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


