A tandem strategy for the synthesis of 2-aminobenzothiazoles via manganese catalyzed CS bond formation
IF 4.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The first manganese catalyzed tandem methodology for the synthesis of 2-aminobenzothiazoles from 2-bromophenyl isothiocyanate and differently substituted amines has been demonstrated. This protocol employs environmentally benign, cost-effective and readily available MnCl2.4H2O as the catalyst under air. The present strategy exhibits wide substrate scope and affords differently substituted 2-aminobenzothiazoles in moderate to good yields.
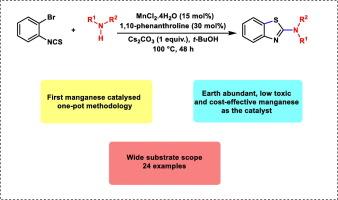
通过锰催化 C S 键形成合成 2-氨基苯并噻唑的串联策略
首次展示了以 2-溴苯基异硫氰酸酯和不同取代胺为原料,通过锰催化串联合成 2-氨基苯并噻唑的方法。该方法在空气中使用对环境无害、成本效益高且易于获得的 MnCl2.4H2O 作为催化剂。本策略具有广泛的底物范围,并能以中等至良好的产率获得不同取代的 2-氨基苯并噻唑。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Communications
化学-物理化学
CiteScore
6.20
自引率
2.70%
发文量
183
审稿时长
46 days
期刊介绍:
Catalysis Communications aims to provide rapid publication of significant, novel, and timely research results homogeneous, heterogeneous, and enzymatic catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


